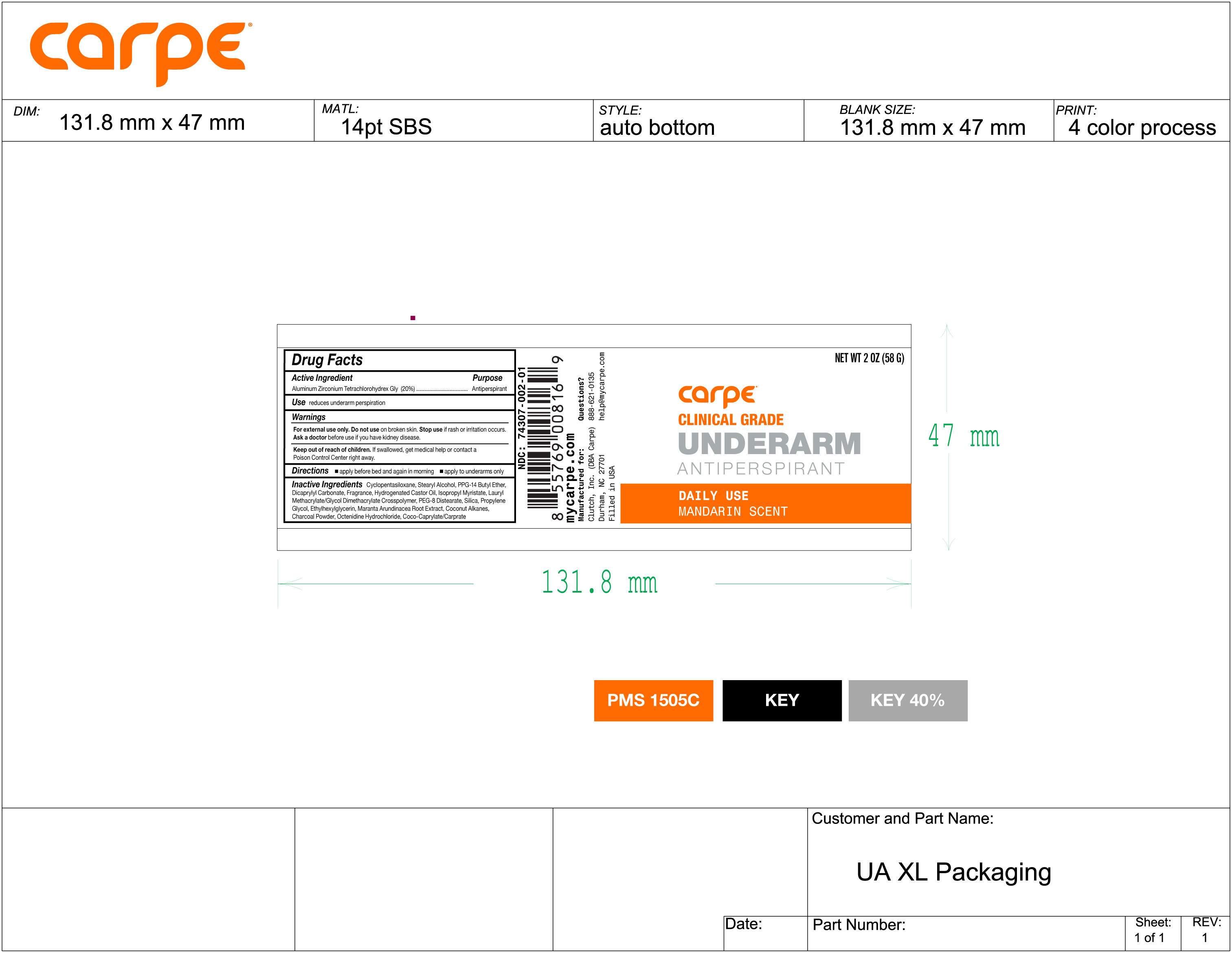
Label: UNDERARM ANTIPERSPIRANT DAILY USE MANDARIN SCENT- aluminum zirconium tetrachlorohydrex gly stick
- NDC Code(s): 74307-002-01
- Packager: Clutch Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive Ingredients
Cyclopentasiloxane, Stearyl Alcohol, PPG-14 Butyl Ether, Dicaprylyl Carbonate, Fragrance, Hydrogenated Castor Oil,
Isopropyl Myristate, Lauryl Methacrylate/Glycol Dimethacrylate Crosspolymer, PEG-8 Distearate, Silica, Propylene Glycol, Ethylhexylglycerin, Maranta Arundinacea Root Extract, Coconut Alkanes, Charcoal Powder, Octenidine Hydrochloride, Coco-Caprylate/Caprate. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDERARM ANTIPERSPIRANT DAILY USE MANDARIN SCENT
aluminum zirconium tetrachlorohydrex gly stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74307-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY (UNII: 8O386558JE) (ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY - UNII:8O386558JE) ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY 20 g in 100 g Inactive Ingredients Ingredient Name Strength ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PPG-14 BUTYL ETHER (UNII: R199TJT95T) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LAURYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EX0F4CZ66H) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) MARANTA ARUNDINACEA ROOT (UNII: FVN346W31A) OCTENIDINE HYDROCHLORIDE (UNII: U84956NU4B) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) PEG-8 DISTEARATE (UNII: 7JNC8VN07M) COCONUT ALKANES (UNII: 1E5KJY107T) ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74307-002-01 58 g in 1 PACKAGE; Type 0: Not a Combination Product 04/23/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 04/23/2020 Labeler - Clutch Inc (080214892) Establishment Name Address ID/FEI Business Operations Wasatch Product Development, LLC. 962452533 manufacture(74307-002)