Label: CISPLATIN injection, solution
- NDC Code(s): 0703-5747-11, 0703-5748-11
- Packager: Teva Parenteral Medicines, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 30, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CISPLATIN INJECTION safely and effectively.
See full prescribing information for CISPLATIN INJECTION.
CISPLATIN injection, for intravenous use
Initial U.S. Approval: 1978WARNING: NEPHROTOXICITY, PERIPHERAL NEUROPATHY, NAUSEA AND VOMITING, and MYELOSUPPRESSION
See full prescribing information for complete boxed warning.
- Nephrotoxicity: Cisplatin Injection can cause severe renal toxicity, including acute renal failure. Ensure adequate hydration. Consider dose reductions or alternative treatments in patients with renal impairment. (2.1, 5.1)
- Peripheral Neuropathy: Cisplatin Injection can cause dose-related peripheral neuropathy. (5.2)
- Nausea and Vomiting: Cisplatin Injection can cause severe nausea and vomiting. Premedicate with antiemetics. (2.1, 5.3)
- Myelosuppression: Cisplatin Injection can cause severe myelosuppression with fatalities due to infections. Monitor blood counts and interrupt therapy accordingly. (5.4)
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Administer pre-treatment hydration and pre- and post-treatment antiemetics. (2.1)
- Cisplatin Injection has been administered intravenously at:
Refer to current treatment guidelines for specific dosing information.
- Administer by slow intravenous infusion. Avoid contact of Cisplatin Injection with aluminum parts. (2.6)
DOSAGE FORMS AND STRENGTHS
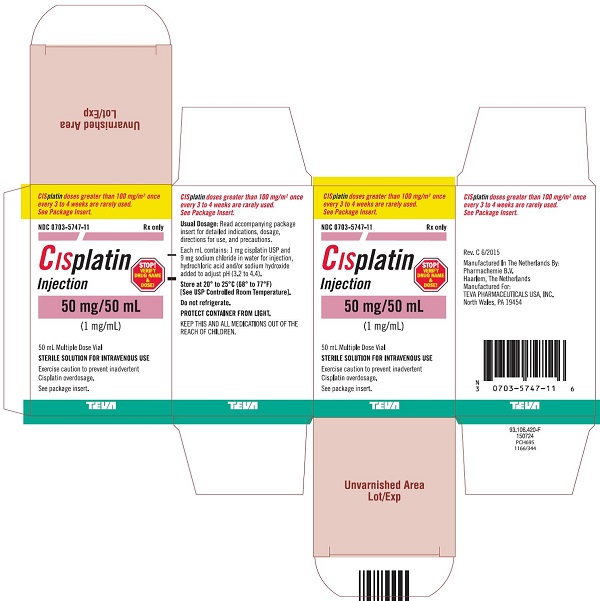
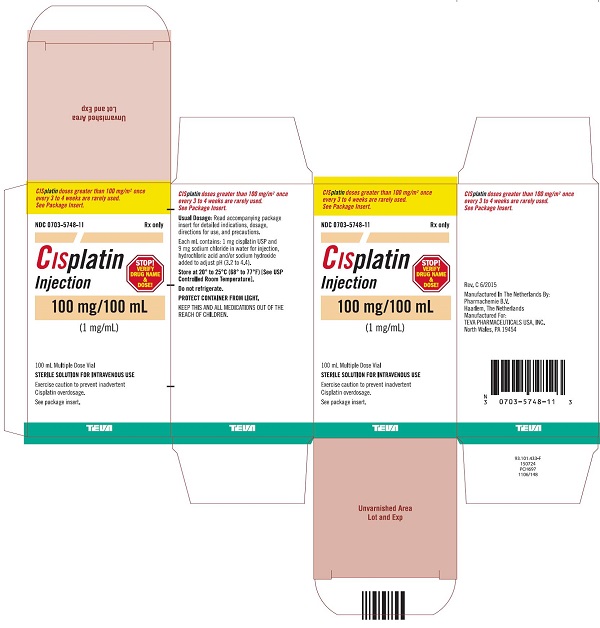
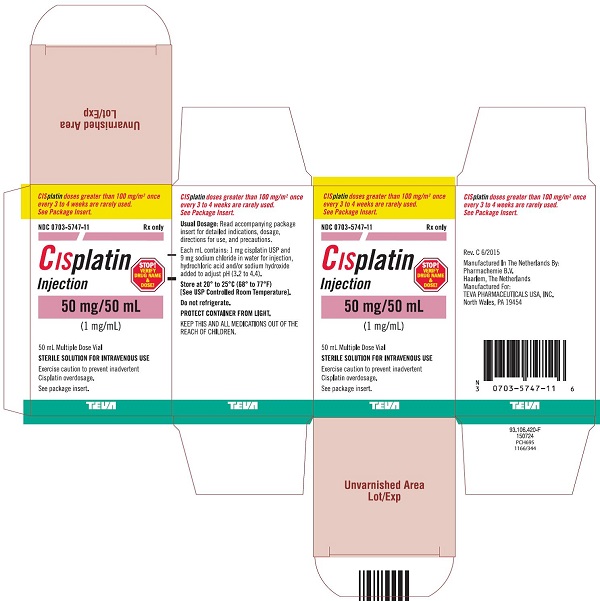
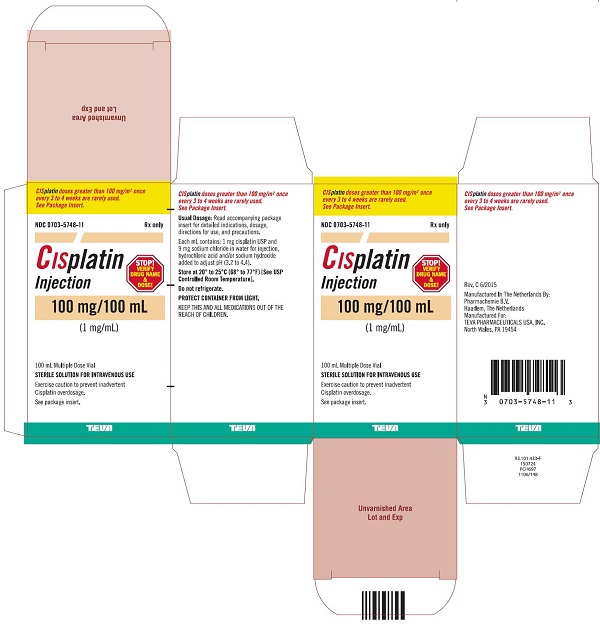
Injection: 50 mg/50 mL (1 mg/mL) and 100 mg/100 mL (1 mg/mL) in Multiple-Dose vials (3)
CONTRAINDICATIONS
Severe hypersensitivity to cisplatin (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions: Anaphylaxis and death may occur; monitor for and treat accordingly (5.5)
- Ototoxicity: Cumulative toxicity may be severe particularly in pediatric patients; consider audiometric and vestibular function monitoring (5.6, 8.4)
- Ocular toxicity: Optic neuritis, papilledema, and cortical blindness may occur (5.7)
- Secondary leukemia: Secondary acute leukemia may occur (5.8)
- Embryo-fetal toxicity: Can cause fetal harm. Advise of potential risk to a fetus and use of effective contraception. (5.9, 8.1, 8.3)
ADVERSE REACTIONS
Common adverse reactions are nephrotoxicity, peripheral neuropathy, nausea and vomiting, myelosuppression, and ototoxicity. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Teva at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: NEPHROTOXICITY, PERIPHERAL NEUROPATHY, NAUSEA AND VOMITING and MYELOSUPPRESSION
1 INDICATIONS AND USAGE
1.1 Advanced Testicular Cancer
1.2 Advanced Ovarian Cancer
1.3 Advanced Bladder Cancer
2 DOSAGE AND ADMINISTRATION
2.1 Hydration and Anti-Emetic Treatment
2.2 Advanced Testicular Cancer
2.3 Advanced Ovarian Cancer
2.4 Advanced Bladder Cancer
2.5 Dose Modifications for Renal Impairment
2.6 Preparation, Handling, and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Nephrotoxicity
5.2 Peripheral Neuropathy
5.3 Nausea and Vomiting
5.4 Myelosuppression
5.5 Hypersensitivity Reactions
5.6 Ototoxicity
5.7 Ocular Toxicity
5.8 Secondary Malignancies
5.9 Embryo-Fetal Toxicity
5.10 Injection Site Reactions
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Use in Patients with Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: NEPHROTOXICITY, PERIPHERAL NEUROPATHY, NAUSEA AND VOMITING and MYELOSUPPRESSION
- Nephrotoxicity: Cisplatin Injection can cause severe renal toxicity, including acute renal failure. Severe renal toxicities are dose-related and cumulative. Ensure adequate hydration and monitor renal function and electrolytes. Consider dose reductions or alternative treatments in patients with renal impairment [see Dosage and Administration (2.1) and Warnings and Precautions (5.1)].
- Peripheral Neuropathy: Cisplatin Injection can cause dose-related peripheral neuropathy that becomes more severe with repeated courses of the drug [see Warnings and Precautions (5.2)].
- Nausea and Vomiting: Cisplatin Injection can cause severe nausea and vomiting. Use highly effective antiemetic premedication [see Dosage and Administration (2.1) and Warnings and Precautions (5.3)].
- Myelosuppression: Cisplatin Injection can cause severe myelosuppression with fatalities due to infections. Monitor blood counts accordingly. Interruption of therapy may be required [see Warnings and Precautions (5.4)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Hydration and Anti-Emetic Treatment
Patients treated with Cisplatin Injection must receive appropriate pre-treatment hydration. Maintain adequate hydration and urinary output for 24 hours after Cisplatin Injection administration [see Warnings and Precautions (5.1)]. Administer pre-treatment and post-treatment antiemetics as appropriate [see Warnings and Precautions (5.7)].
2.2 Advanced Testicular Cancer
Cisplatin Injection has been administered at 20 mg/m2 intravenously daily for 5 days per cycle. Other doses and combination regimens have been used.
2.3 Advanced Ovarian Cancer
Cisplatin Injection has been administered at 75 mg/m2 to 100 mg/m2 intravenously per cycle once every 3 to 4 weeks on Day 1. Other doses and combination regimens have been used.
2.4 Advanced Bladder Cancer
Cisplatin Injection has been administered at 50 mg/m2 to 70 mg/m2 intravenously per cycle once every 3 to 4 weeks. For heavily pretreated patients, an initial dose of 50 mg/m2 per cycle repeated every 4 weeks is recommended. Other doses and combination in regimens have been used.
2.5 Dose Modifications for Renal Impairment
Consider alternative treatments or dose reductions for patients with impaired creatinine clearance, myelosuppression, or neuropathy. Consider permanent discontinuation for Grade 3-4 neuropathy [See Warnings and Precautions (5.1)].
2.6 Preparation, Handling, and Administration
Do not use needles or intravenous sets containing aluminum parts that can come in contact with Cisplatin Injection during preparation or administration. Aluminum reacts with Cisplatin Injection, causing precipitate formation and a loss of potency.
Cisplatin Injection is a cytotoxic drug. Follow applicable special handling and disposable procedures.1
Instructions for Preparation
Pretreatment hydration with 1 to 2 liters of fluid infused for 8 to 12 hours prior to a Cisplatin Injection dose is recommended. The drug is then diluted in 2 liters of 5% Dextrose in 1/2 or 1/3 normal saline containing 37.5 g of mannitol, and infused over a 6- to 8-hour period. If diluted solution is not to be used within 6 hours, protect solution from light. Do not dilute Cisplatin Injection in just 5% Dextrose Injection. Adequate hydration and urinary output must be maintained during the following 24 hours.
A repeat course of Cisplatin Injection should not be given until the serum creatinine is below 1.5 mg/100 mL, and/or the BUN is below 25 mg/100 mL. A repeat course should not be given until circulating blood elements are at an acceptable level (platelets ≥ 100,000/mm3, WBC ≥ 4000/mm3). Subsequent doses of Cisplatin Injection should not be given until an audiometric analysis indicates that auditory acuity is within normal limits.
The aqueous solution should be used intravenously only and should be administered by intravenous infusion over a 6- to 8-hour period [see Dosage and Administration (2)].
Handling
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
NOTE TO PHARMACIST: Exercise caution to prevent inadvertent cisplatin overdosage. Please call prescriber if dose is greater than 100 mg/m2 per cycle. Aluminum flip-off seal of vial have been imprinted with the following statement: CALL DR. IF DOSE > 100 MG/M2/CYCLE.
Administration
Administer Cisplatin Injection by slow intravenous infusion. CISPLATIN SHOULD NOT BE GIVEN BY RAPID INTRAVENOUS INJECTION.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Cisplatin Injection is contraindicated in patients with severe hypersensitivity to cisplatin [see Warnings and Precautions (5.4)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Nephrotoxicity
Cisplatin Injection can cause dose-related nephrotoxicity, including acute renal failure that becomes more prolonged and severe with repeated courses of the drug. Renal toxicity typically begins during the second week after a dose of Cisplatin Injection. Patients with baseline renal impairment, geriatric patients, patients who are taking other nephrotoxic drugs, or patients who are not well hydrated may be more susceptible to nephrotoxicity [see Use in Specific Populations (8.5, 8.6)].
Ensure adequate hydration before, during, and after Cisplatin Injection administration [see Dosage and Administration (2.1)]. Measure serum creatinine, blood urea nitrogen, creatinine clearance, and serum electrolytes including magnesium prior to initiating therapy, and as clinically indicated. Consider magnesium supplementation as clinically needed.
Consider alternative treatments or reduce the dose of Cisplatin Injection for patients with baseline renal impairment or who develop significant reductions in creatinine clearance during treatment with Cisplatin Injection according to clinical treatment guidelines [see Dosage and Administration (2.5)].
5.2 Peripheral Neuropathy
Cisplatin Injection can cause dose-related peripheral neuropathy that becomes more severe with repeated courses of the drug. Neurologic symptoms have been reported to occur after a single dose. Neuropathy can also have a delayed onset from 3 to 8 weeks after the last dose of Cisplatin Injection. Manifestations include paresthesias in a stocking-glove distribution, areflexia, and loss of proprioception and vibratory sensation. The neuropathy may progress further even after stopping treatment. Peripheral neuropathy may be irreversible in some patients.
Perform a neurological examination before initiating Cisplatin Injection, at appropriate intervals during therapy, and after completion of therapy. Consider discontinuation of Cisplatin Injection for patients who develop symptomatic peripheral neuropathy. Geriatric patients may be more susceptible to peripheral neuropathy [see Use in Specific Populations (8.5)].
5.3 Nausea and Vomiting
Cisplatin Injection is a highly emetogenic antineoplastic agent. Premedicate with anti-emetic agents [see Dosage and Administration (2.1)]. Without antiemetic therapy, marked nausea and vomiting occur in almost all patients treated with Cisplatin Injection and may be so severe that the drug must be discontinued. Nausea and vomiting may begin within 1 to 4 hours after treatment and last up to 72 hours. Maximal intensity occurs 48 to 72 hours after administration.
Various degrees of vomiting, nausea, and/or anorexia may persist for up to 1 week after treatment. Delayed nausea and vomiting (begins or persists 24 hours or more after chemotherapy) has occurred in patients attaining complete emetic control on the day of Cisplatin Injection therapy. Consider the use of additional anti-emetics following infusion.
5.4 Myelosuppression
Myelosuppression suppression occurs in 25% to 30% of patients treated with Cisplatin Injection. Fever and infection have been reported in patients with neutropenia. Potential fatalities due to infection (secondary to myelosuppression) have been reported. Geriatric patients may be more susceptible to myelosuppression [see Use in Specific Populations (8.5)].
Perform standard hematologic tests before initiating Cisplatin Injection, before each subsequent course, and as clinically indicated. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with Cisplatin Injection. For patients who develop severe myelosuppression during treatment with Cisplatin Injection, consider dose modifications and manage according to clinical treatment guidelines.
5.5 Hypersensitivity Reactions
Cisplatin Injection can cause severe hypersensitivity reactions, including anaphylaxis and death. Manifestations have included facial edema, wheezing, tachycardia, and hypotension. Hypersensitivity reactions have occurred within minutes of administration to patients with prior exposure to Cisplatin Injection.
Monitor patients receiving Cisplatin Injection for possible hypersensitivity reactions. Ensure supportive equipment and medications are available to treat severe hypersensitivity reactions. Severe hypersensitivity reactions require immediate discontinuation of Cisplatin Injection and aggressive therapy. Patients with a history of severe hypersensitivity reactions should not be rechallenged with Cisplatin Injection [see Contraindications (4)]. Cross-reactivity between platinum-based antineoplastic agents has been reported. Cases of severe hypersensitivity reactions have recurred after rechallenging patients with a different platinum agent.
5.6 Ototoxicity
Cisplatin Injection can cause ototoxicity, which is cumulative and may be severe. Consider audiometric and vestibular function monitoring.
Ototoxicity is manifested by tinnitus, hearing loss in the high frequency range (4,000 to 8,000 Hz) and/or decreased ability to hear normal conversational tones. Ototoxicity can occur during or after treatment and can be unilateral or bilateral. Deafness after the initial dose of Cisplatin Injection has been reported. Vestibular toxicity has also been reported.
Ototoxic effects can be more severe and detrimental in pediatric patients, particularly in patients less than 5 years of age. The prevalence of hearing loss in pediatric patients is estimated to be 40-60%. Additional risk factors for ototoxicity include simultaneous cranial irradiation, treatment with other ototoxic drugs and renal impairment. Consider audiometric and vestibular testing in all pediatric patients receiving cisplatin [see Use in Specific Populations (8.4)].
Genetic factors (e.g. variants in the thiopurine S-methyltransferase [TPMT] gene) may also contribute to the cisplatin-induced ototoxicity; although this association has not been consistent across populations and study designs.
5.7 Ocular Toxicity
Optic neuritis, papilledema, and cortical blindness have been reported in patients receiving standard recommended doses of Cisplatin Injection. Blurred vision and altered color perception have been reported after the use of regimens with higher doses and dose frequencies of Cisplatin Injection. The altered color perception manifests as a loss of color discrimination, particularly in the blue-yellow axis and irregular retinal pigmentation of the macular area on fundoscopic exam. Improvement and/or total recovery usually occurs after discontinuing Cisplatin Injection but can be delayed.
5.8 Secondary Malignancies
The development of acute leukemia secondary to the use of Cisplatin Injection has been reported. In these reports, Cisplatin Injection was generally given in combination with other leukemogenic agents.
5.9 Embryo-Fetal Toxicity
Based on human data, Cisplatin Injection can cause fetal harm when administered to a pregnant woman. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for 14 months after the last dose of Cisplatin Injection. Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 11 months after the last dose of Cisplatin Injection [see Use in Specific Populations (8.1, 8.3)].
5.10 Injection Site Reactions
Injection site reactions can occur during the administration of Cisplatin Injection. Local soft tissue toxicity has been reported following extravasation of Cisplatin Injection. Severity of the local tissue toxicity appears to be related to the concentration of the Cisplatin Injection solution. Infusion of solutions with a Cisplatin Injection concentration greater than 0.5 mg/mL may result in tissue cellulitis, fibrosis, necrosis, pain, edema, and erythema.
Because of the possibility of extravasation, closely monitor the infusion site during drug administration.
-
6 ADVERSE REACTIONS
The following adverse reactions are described in greater detail, in other sections:
- Nephrotoxicity [see Warnings and Precautions (5.1)]
- Peripheral Neuropathy [see Warnings and Precautions (5.2)]
- Nausea and vomiting [see Warnings and Precautions (5.3)]
- Myelosuppression [see Warnings and Precautions (5.4)]
- Hypersensitivity reactions [see Warnings and Precautions (5.5)]
- Ototoxicity [see Warnings and Precautions (5.6]
- Ocular toxicity [see Warnings and Precautions (5.7)]
- Secondary malignancies [see Warnings and Precautions (5.8)]
- Injection site reactions [see Warnings and Precautions (5.10)]
Common adverse reactions are nephrotoxicity, peripheral neuropathy, nausea and vomiting myelosuppression, and ototoxicity. The following adverse reactions have been identified from clinical trials or post-marketing surveillance.
Blood and lymphatic system disorders: Coombs-positive hemolytic anemia. hemolytic uremic syndrome, thrombotic thrombocytopenic purpura
Cardiovascular disorders: Venous thromboembolism, arterial thromboembolism, myocardial infarction, cerebrovascular accident, thrombotic microangiopathy, cerebral arteritis, pericardial effusion, cardiac failure, ventricular dysfunction, Raynaud’s phenomenon
Eye disorders: Optic neuritis, papilledema, cortical blindness, blurred vision, color blindness, retinal pigmentation
Gastrointestinal disorders: Nausea, vomiting, anorexia, diarrhea, stomatitis, gastrointestinal perforation, pancreatitis, hiccups
General disorders: Asthenia, malaise
Hepatobiliary disorders: Elevations of aminotransferases, lactate dehydrogenase, and bilirubin; hepatic failure
Hypersensitivity: Anaphylaxis, facial edema, wheezing, tachycardia, and hypotension
Local Site Reactions: Tissue cellulitis, fibrosis, necrosis, pain, edema, and erythema
Metabolism and nutrition disorders: Hypomagnesemia, often requiring magnesium supplementation; hyperuricemia, other electrolyte abnormalities (hypocalcemia, hyponatremia, hypokalemia, and hypophosphatemia), Syndrome of Inappropriate Antidiuretic Hormone Excretion (SIADH), dehydration, tumor lysis syndrome, increased serum amylase
Musculoskeletal disorders: Muscle cramps (localized, painful, involuntary skeletal muscle contractions of sudden onset and short duration)
Nervous system disorders: Peripheral neuropathy, Encephalopathy, loss of motor function, loss of taste, leukoencephalopathy, reversible posterior leukoencephalopathy syndrome, progressive multifocal leukoencephalopathy, seizures, Lhermitte’s sign, dorsal column myelopathy, autonomic neuropathy, seizures, involuntary skeletal muscle contractions, tetany (with hypocalcemia and hypomagnesemia)
Ototoxicity: Tinnitus, hearing loss, deafness, vestibular toxicity
Renal and urinary disorders: Nephrotoxicity including renal failure, renal electrolyte wasting, azotemia, decreased creatinine clearance
Respiratory disorders: pneumonitis/interstitial lung disease, pulmonary embolism
Skin and subcutaneous tissue disorders: Alopecia, rash
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on human data from published literature, Cisplatin Injection can cause fetal harm when administered to pregnant women. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Data demonstrates transplacental transfer of cisplatin. Exposure of pregnant women to cisplatin-containing chemotherapy has been associated with oligohydramnios, intrauterine growth restriction, and preterm birth. Cases of neonatal acute respiratory distress syndrome, cytopenias, and hearing loss have been reported. Cisplatin Injection administration to animals during and after organogenesis resulted in teratogenicity. A published study in mice showed placental transfer of cisplatin increased with placenta maturation.
The background risk of major birth defects and miscarriage for the indicated populations are unknown. However, the background risk in the U.S. general population of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
8.2 Lactation
Risk Summary
Limited data from published literature report the presence of cisplatin in human milk in low amounts. Because of the potential for serious adverse reactions from Cisplatin Injection in a breastfed child and because of the potential for tumorigenicity shown for Cisplatin Injection, advise lactating women not to breastfeed during treatment with Cisplatin Injection.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiation of Cisplatin Injection.Contraception
Females
Cisplatin Injection can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment and for 14 months following the last dose of Cisplatin Injection.Males
Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 11 months after the last dose of Cisplatin Injection.Infertility
Females
The use of cisplatin has been associated with cumulative dose-dependent ovarian failure, premature menopause, and reduced fertility.Males
The use of cisplatin has been associated with a cumulative dose-dependent impairment of spermatogenesis (oligospermia, azoospermia; possibly irreversible) and reduced fertility.8.4 Pediatric Use
Ototoxic effects may be more severe and detrimental in pediatric patients receiving Cisplatin Injection, particularly in patients less than 5 years of age. Consider audiometric and vestibular function monitoring in all patients receiving Cisplatin Injection. The prevalence of hearing loss in pediatric patients is particularly high and is estimated to be 40% to 60%.
Earlier detection of hearing loss can limit the potential impact of hearing impairment on a pediatric patient’s cognitive and social development [see Warnings and Precautions (5.6)].
8.5 Geriatric Use
For the treatment of metastatic testicular tumors or advanced bladder cancer, clinical studies of Cisplatin Injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In four clinical trials of combination chemotherapy for advanced ovarian carcinoma, 1,484 patients received cisplatin either in combination with cyclophosphamide or with paclitaxel. Of these, 426 (29%) were older than 65 years. In these trials, age was not found to be a prognostic factor for survival. However, in a later secondary analysis for one of these trials, geriatric patients were found to have shorter survival compared with younger patients.
In all four trials, geriatric patients experienced more severe neutropenia than did younger patients. Higher incidences of severe thrombocytopenia and leukopenia were also seen in geriatric patients compared with younger patients, although not in all cisplatin-containing treatment arms. In the two trials where nonhematologic toxicity was evaluated according to age, geriatric patients had a numerically higher incidence of peripheral neuropathy than did younger patients. Other reported clinical experience suggests that geriatric patients may be more susceptible to nephrotoxicity, myelosuppression, and infectious complications than are younger patients [see Warnings and Precautions (5.1, 5.2, 5.4)].
Cisplatin is known to be substantially excreted by the kidney. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and renal function should be monitored.
8.6 Use in Patients with Renal Impairment
Patients with baseline renal impairment may be more susceptible to nephrotoxicity [see Warnings and Precautions (5.1)]. Ensure adequate hydration before, during, and after Cisplatin Injection administration [see Dosage and Administration (2.1)]. Measure serum creatinine, blood urea nitrogen, creatinine clearance, and serum electrolytes prior to initiating therapy, and as clinically indicated. Consider alternative treatments or reduce the dose of Cisplatin Injection for patients with baseline renal impairment or who develop significant reductions in creatinine clearance during treatment with Cisplatin Injection according to clinical treatment guidelines [see Dosage and Administration (2.5)].
-
10 OVERDOSAGE
Acute overdosage with Cisplatin Injection may result in renal failure, hepatic failure, hearing loss, ocular toxicity, myelosuppression, nausea and vomiting, and neuritis. In addition, death can occur following overdosage.
Management of overdosage should include general supportive measures to sustain the patient through any period of toxicity that may occur. Important measures include renal protection by intravenous hydration with or without the use of an osmotic diuretic. Hemodialysis is not effective because of the high degree of protein binding of Cisplatin Injection. Plasmapheresis has been used to treat cases of Cisplatin Injection overdosage, but the optimal treatment regimen has not been established.
For current information on the management of poisoning or overdosage, contact the National Poison Control Center at 1-800-222-1222 or www.poison.org.
-
11 DESCRIPTION
Cisplatin Injection, a platinum-based drug for intravenous use, is a clear, light yellow sterile aqueous solution. Each mL of Cisplatin Injection contains: 1 mg cisplatin USP, 9 mg sodium chloride, hydrochloric acid and/or sodium hydroxide to adjust pH, and Water for Injection to a final volume of 50 mL or 100 mL. The pH range of Cisplatin Injection is 3.2 to 4.4.
Cisplatin USP, the active ingredient in Cisplatin Injection, is an orange-yellow crystalline powder with the molecular formula Cl2H6N2Pt and a molecular weight of 300.05. Cisplatin, USP is a heavy metal complex containing a central atom of platinum surrounded by two chloride atoms and two ammonia molecules in the cis position. It is soluble in water or saline at 1 mg/mL and in dimethylformamide at 24 mg/mL. It has a melting point of 207°C.
The structural formula is:
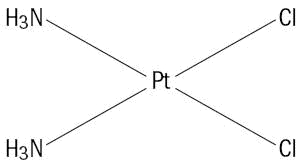
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The main mechanism of the cytotoxic action involves the binding of cisplatin to genomic DNA in the cell nucleus to form interstrand and intrastrand cross-links. This interferes with normal transcription and/or DNA replication mechanisms and triggers cytotoxic processes that lead to cell death.
12.3 Pharmacokinetics
Distribution
Cisplatin dose not undergo the instantaneously and reversible binding to plasma protein that is characteristic of normal drug-protein binding. Platinum from cisplatin, but not cisplatin itself, becomes bound to several plasma proteins, including albumin, transferrin, and gamma globulin. Three hours after a bolus injection and 2 hours after the end of a 3-hour infusion, 90% of the plasma platinum is protein bound. The complexes between albumin and the platinum from cisplatin do not dissociate to a significant extent and are slowly eliminated with a minimum half-life of 5 days or more.Following cisplatin doses of 20 mg/m2 to 120 mg/m2, platinum is present in tissues for as long as 180 days after the last administration. With the exception of intracerebral tumors, platinum concentrations in tumors are generally somewhat lower than the concentrations in the organ where the tumor is located. Hepatic metastases have the highest platinum concentrations, but these are similar to the platinum concentrations in normal liver. Maximum red blood cell concentrations of platinum are reached within 90 to 150 minutes after a 100 mg/m2 dose of cisplatin and decline in a biphasic manner with a terminal half-life of 36 to 47 days.
Metabolism
The chlorine atoms of cisplatin are more subject to chemical displacement reactions by nucleophiles, such as water or sulfhydryl groups, than to enzyme-catalyzed metabolism. At physiological pH, the predominant molecular species are cisplatin and monohydroxymonochloro cis-diamine platinum (II) in nearly equal concentrations. The latter, combined with the possible direct displacement of the chlorine atoms by sulfhydryl groups of amino acids or proteins, accounts for the instability of cisplatin in biological matrices. The ratios of cisplatin to total free (ultrafilterable) platinum in the plasma vary considerably between patients and range from 0.5 to 1.1 after a dose of 100 mg/m2.Elimination
Over a dose range of 40 mg to 140 mg cisplatin per m2 given as a bolus injection or as infusions varying in length from 1 hour to 24 hours, from 10% to about 40% of the administered platinum is excreted in the urine in 24 hours. Over 5 days following administration of 40 mg/m2 to 100 mg/m2 doses given as rapid, 2- to 3-hour or 6- to 8-hour infusions, a mean of 35% to 51% of the dosed platinum is excreted in the urine. Similar mean urinary recoveries of platinum of about 14% to 30% of the dose are found following 5 daily administrations of 20 mg/m2 per day, 30 mg/m2 per day, or 40 mg/m2 per day. Only a small percentage of the administered platinum is excreted beyond 24 hours post-infusion and most of the platinum excreted in the urine in 24 hours is excreted within the first few hours.The parent compound, cisplatin, is excreted in the urine and accounts for 13% to 17% of the dose excreted within 1 hour after administration of 50 mg/m2. The mean renal clearance of cisplatin exceeds creatinine clearance and was 62 mL/min per m2 and 50 mL/min per m2 following administration of 100 mg/m2 as 2-hour or 6- to 7-hour infusions, respectively.
Plasma concentrations of the parent compound, cisplatin, decrease monoexponentially with a half-life of about 20 to 30 minutes following bolus administrations of 50 mg/m2 or 100 mg/m2 doses. Monoexponential decreases and plasma half-lives of about 0.5 hour are also seen following 2-hour or 7-hour infusions of 100 mg/m2. After the latter, the total body clearances and volumes of distribution at steady-state for cisplatin are about 15 Liters per hour per m2 to 16 Liters per hour per m2 and 11 Liters per m2 to 12 Liters per m2.
The renal clearance of free (ultrafilterable) platinum also exceeds the glomerular filtration rate, indicating that cisplatin or other platinum-containing molecules are actively secreted by the kidneys. The renal clearance of free platinum is nonlinear and variable and is dependent on dose, urine flow rate, and individual variability in the extent of active secretion and possible tubular reabsorption.
No significant relationships exist between the renal clearance of either free platinum or cisplatin and creatinine clearance.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic effect of Cisplatin Injection was studied in BDIX rats. Cisplatin Injection was administered three times a week at 1 mg/kg body weight intraperitoneally to 50 BDIX rats for 3 weeks. Four hundred fifty-five days after the first application, 33 animals died, 13 of them related to malignancies (12 leukemias and 1 renal fibrosarcoma) [see Warnings and Precautions (5.8)].
Cisplatin is mutagenic in the bacteria reverse mutation (Ames) test and produces chromosome aberrations in mammalian cells.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Cisplatin Injection, is a clear, light yellow sterile aqueous solution, for intravenous use, supplied as below.
NDC Numbers
Strengths
Size
0703-5747-11
50 mg/50 mL (1 mg/mL)
50 mL Multiple-Dose vial
0703-5748-11
100 mg/100 mL (1 mg/mL)
100 mL Multiple-Dose vial
Storage
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Do not refrigerate. Protect container from light.
The cisplatin remaining in the amber vial following initial entry is stable for 28 days protected from light or for 7 days under fluorescent room light.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Handling and Disposal
Cisplatin Injection is a cytotoxic drug. Follow applicable special handling and disposal procedures.1 -
17 PATIENT COUNSELING INFORMATION
Nephrotoxicity
Inform patients that Cisplatin Injection can cause nephrotoxicity and that renal function and electrolyte monitoring during treatment is necessary. If indicated, inform patients about the use of electrolyte supplements [see Warnings and Precautions (5.1)].Peripheral Neuropathy
Advise patients to report any new paresthesias to their healthcare provider [see Warnings and Precautions (5.2)].Nausea and Vomiting
Advise patients concerning the use of antiemetics to prevent nausea and vomiting and to report persistent or severe symptoms to their healthcare provider [see Warnings and Precautions (5.3)].Myelosuppression
Advise patients that Cisplatin Injection can reduce the absolute neutrophil count and the platelet count resulting in an increased risk of infection and bleeding and to contact their healthcare provider for new onset fever, symptoms of infection, or bleeding [see Warnings and Precautions (5.4)].Ototoxicity
Advise patients to report any symptoms of hearing loss or vestibular dysfunction to their healthcare provider and that periodic monitoring of hearing may be performed [see Warnings and Precautions (5.6)].Embryo-Fetal Toxicity
- Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider if they are pregnant or become pregnant [see Warnings and Precautions 5.9 and Use in Specific Populations 8.1)].
- Advise females of reproductive potential to use effective contraception during treatment and for 14 months following the last dose of Cisplatin Injection [see Use in Specific Populations (8.3)].
- Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 11 months following the last dose of Cisplatin Injection [see Use in Specific Populations (8.3)].
Lactation
Advise females not to breastfeed during treatment with Cisplatin Injection [see Use in Specific Populations (8.2)].Infertility
Inform patients that treatment with Cisplatin Injection may lead to permanent impairment of spermatogenesis, ovarian failure or premature menopause, and reduced fertility in both genders [see Use in Specific Populations (8.3)].Alopecia
Inform patients that Cisplatin Injection can cause alopecia.Manufactured for:
Teva Pharmaceuticals USA, Inc.,
North Wales, PA 19454Rev. D 8/2022
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CISPLATIN
cisplatin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0703-5747 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CISPLATIN (UNII: Q20Q21Q62J) (CISPLATIN - UNII:Q20Q21Q62J) CISPLATIN 50 mg in 50 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0703-5747-11 1 in 1 CARTON 06/01/2000 1 50 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074656 06/01/2000 CISPLATIN
cisplatin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0703-5748 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CISPLATIN (UNII: Q20Q21Q62J) (CISPLATIN - UNII:Q20Q21Q62J) CISPLATIN 100 mg in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0703-5748-11 1 in 1 CARTON 06/01/2000 1 100 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074656 06/01/2000 Labeler - Teva Parenteral Medicines, Inc. (794362533)