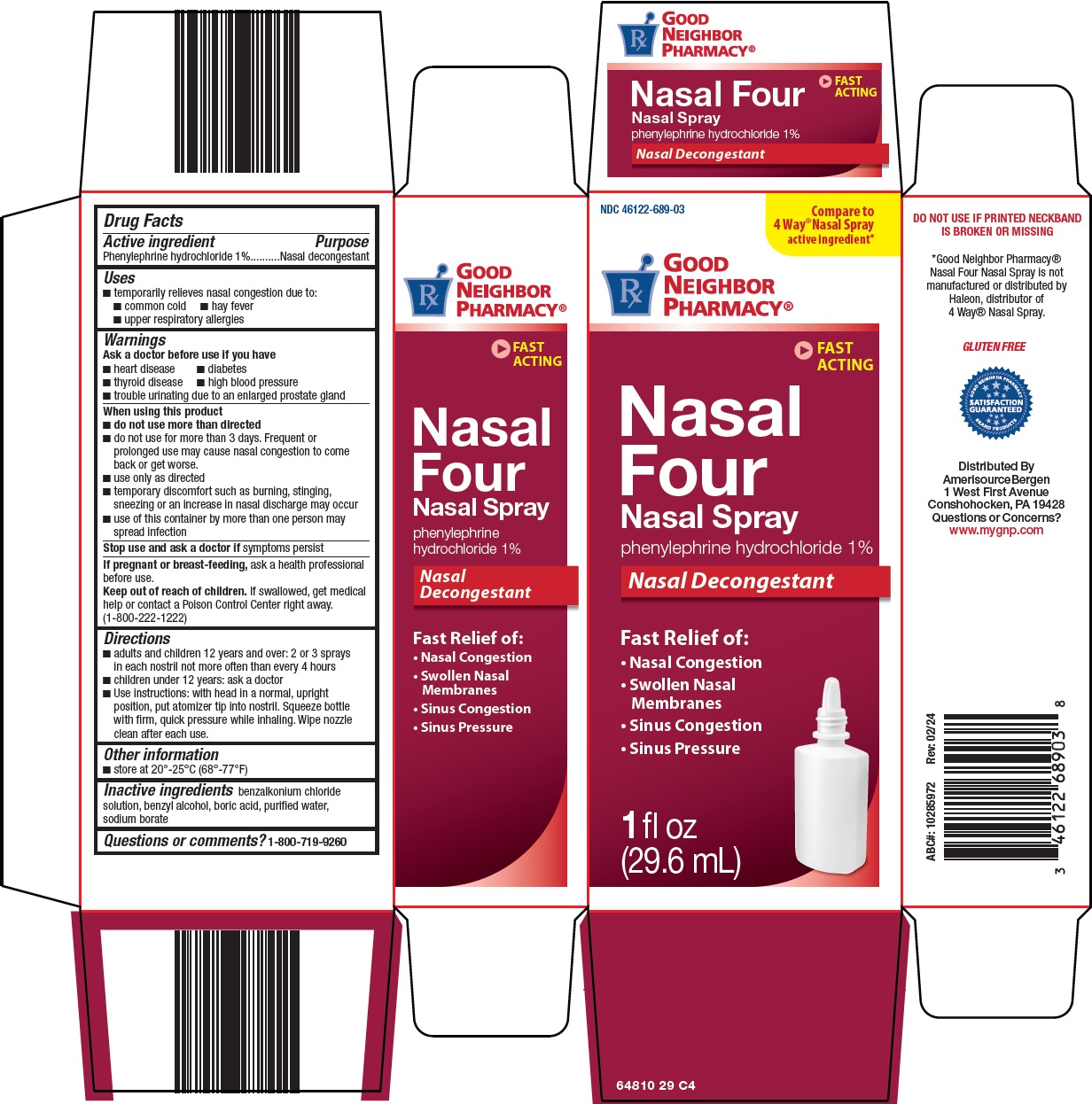
Label: GOOD NEIGHBOR PHARMACY NASAL FOUR- phenylephrine hydrochloride spray
- NDC Code(s): 46122-689-03
- Packager: Amerisource Bergen
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- •
- heart disease
- •
- diabetes
- •
- thyroid disease
- •
- high blood pressure
- •
- trouble urinating due to an enlarged prostate gland
When using this product
- •
- do not use more than directed
- •
- do not use for more than 3 days. Frequent or prolonged use may cause nasal congestion to come back or get worse.
- •
- use only as directed
- •
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- •
- use of this container by more than one person may spread infection
-
Directions
- •
- adults and children 12 years and over: 2 or 3 sprays in each nostril not more often than every 4 hours
- •
- children under 12 years: ask a doctor
- •
- Use instructions: with head in a normal, upright position, put atomizer tip into nostril. Squeeze bottle with firm, quick pressure while inhaling. Wipe nozzle clean after each use.
- Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
GOOD NEIGHBOR PHARMACY NASAL FOUR
phenylephrine hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46122-689 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BENZYL ALCOHOL (UNII: LKG8494WBH) BORIC ACID (UNII: R57ZHV85D4) SODIUM BORATE (UNII: 91MBZ8H3QO) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46122-689-03 1 in 1 CARTON 04/15/2021 1 29.6 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 04/15/2021 Labeler - Amerisource Bergen (007914906)