Label: ARMODAFINIL tablet
- NDC Code(s): 0781-8029-31, 0781-8037-31, 0781-8045-31, 0781-8053-31
- Packager: Sandoz Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated January 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ARMODAFINIL TABLETS safely and effectively. See full prescribing information for ARMODAFINIL TABLETS. ARMODAFINIL tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEArmodafinil tablets are indicated to improve wakefulness in adult patients with excessive sleepiness associated with obstructive sleep apnea (OSA), narcolepsy, or shift work disorder ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage in Obstructive Sleep Apnea (OSA) and Narcolepsy - The recommended dosage of armodafinil tablets for patients with OSA or narcolepsy is 150 mg to 250 mg taken orally once a day as a ...
-
3 DOSAGE FORMS AND STRENGTHS50 mg – round, white to off-white tablet with - on one side and "205" on the other - 150 mg – oval, white to off-white tablet with - on one side and "215" on the other - 200 mg – rounded ...
-
4 CONTRAINDICATIONSArmodafinil is contraindicated in patients with known hypersensitivity to modafinil or armodafinil or its inactive ingredients [see Warnings and Precautions (5.1, 5.2, 5.3)].
-
5 WARNINGS AND PRECAUTIONS5.1 Serious Dermatologic Reactions, including Stevens-Johnson Syndrome and - Toxic Epidermal Necrosis - Serious rash requiring hospitalization and discontinuation of treatment has been reported in ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described below and elsewhere in the labeling: Serious Dermatologic Reactions [see Warnings and Precautions (5.1)] Drug Reaction with Eosinophilia and ...
-
7 DRUG INTERACTIONSEffects of Armodafinil on CYP3A4/5 Substrates - The clearance of drugs that are substrates for CYP3A4/5 (e.g., steroidal contraceptives, cyclosporine, midazolam, and triazolam) may be increased by ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to armodafinil during pregnancy. Healthcare providers are encouraged ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - Armodafinil is a Schedule IV controlled substance. 9.2 Abuse - Abuse of armodafinil has been reported in patients treated with armodafinil. Patterns of abuse have ...
-
10 OVERDOSAGEFatal overdoses involving modafinil alone or involving armodafinil or modafinil in combination with other drugs have been reported in the postmarketing setting. Symptoms most often accompanying ...
-
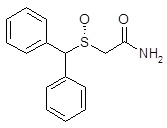
11 DESCRIPTIONArmodafinil tablets are a wakefulness‑promoting agent for oral administration. Armodafinil is the R-enantiomer of modafinil which is a 1:1 mixture of the R- and S-enantiomers. The chemical name ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism(s) through which armodafinil promotes wakefulness is unknown. Armodafinil (R-modafinil) has pharmacological properties similar to those of modafinil (a ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a mouse carcinogenicity study, armodafinil (R-modafinil) was administered at oral doses of up to 300 mg/kg/day in ...
-
14 CLINICAL STUDIES14.1 Obstructive Sleep Apnea (OSA) The effectiveness of armodafinil in improving wakefulness in patients with excessive sleepiness associated with OSA was established in two 12-week ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Armodafinil tablets are available as follows: 50 mg - Each round, white to off-white tablet is debossed with on one side and "205" on the other. NDC 0781-8029-31 – Bottles of ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Serious Dermatologic Reactions - Advise patients and caregivers about the risk of potentially fatal serious skin ...
-
MEDICATION GUIDEArmodafinil tablets, for oral use, C-IV - (are-moe-DAFF-ih-nil) What is the most ...
-
Package/Label Display PanelNDC 0781-8029-31 - Armodafinil Tablets CIV - 50 mg - Rx only - Medication Guide Required: Each time armodafinil tablets are dispensed, give the patient a Medication Guide. 30 Tablets - SANDOZ - S
-
Package/Label Display PanelNDC 0781-8037-31 - Armodafinil Tablets CIV - 150 mg - Rx only - Medication Guide Required: Each time armodafinil tablets are dispensed, give the patient a Medication Guide. 30 Tablets - SANDOZ - S
-
Package/Label Display PanelNDC 0781-8045-31 - Armodafinil Tablets CIV - 200 mg - Rx only - Medication Guide Required: Each time armodafinil tablets are dispensed, give the patient a Medication Guide. 30 ...
-
Package/Label Display PanelNDC 0781-8053-31 - Armodafinil Tablets CIV - 250 mg - Rx only - Medication Guide Required: Each time armodafinil tablets are dispensed, give the patient a Medication Guide. 30 Tablets - SANDOZ - S
-
INGREDIENTS AND APPEARANCEProduct Information









 on one side and "205" on the other.
on one side and "205" on the other. on one side and "215" on the other.
on one side and "215" on the other. on one side and "220" on the other.
on one side and "220" on the other. on one side and "225" on the other.
on one side and "225" on the other.


