Label: BETAMETHASONE VALERATE cream
- NDC Code(s): 68071-5224-5
- Packager: NuCare Pharmaceuticals,Inc.
- This is a repackaged label.
- Source NDC Code(s): 0713-0326
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
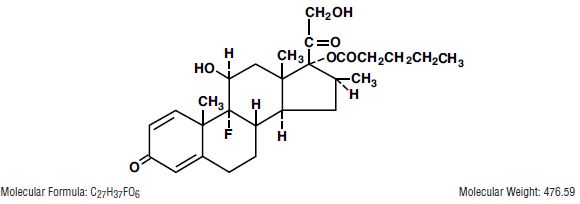
DESCRIPTION Betamethasone Valerate Cream and Ointment contain betamethasone valerate USP, a synthetic adrenocorticosteroid for dermatologic use. Betamethasone, an analog of prednisolone, has a high degree of ...
-
CLINICAL PHARMACOLOGY Topical corticosteroids share anti-inflammatory, antipruritic and vasoconstrictive actions. The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear ...
-
PharmacokineticsThe extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings ...
-
INDICATIONS AND USAGETopical corticosteroids are indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
-
CONTRAINDICATIONSTopical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
- PRECAUTIONS
-
GeneralSystemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome, hyperglycemia, and glucosuria in ...
-
Information for PatientsPatients using topical corticosteroids should receive the following information and instructions: 1. This medication is to be used as directed by the physician.. It is for external ...
-
Laboratory testsThe following tests may be helpful in evaluating the HPA axis suppression: Urinary free cortisol test - ACTH stimulation test
-
Carcinogenesis, Mutagenesis and Impairment of FertilityLong-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of topical corticosteroids. Studies to determine mutagenicity with ...
-
PregnancyTeratogenic Effects — Pregnancy Category C - Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage ...
-
Nursing MothersIt is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered ...
-
Pediatric UsePediatric patients may demonstrate greater susceptibility to topical corticosteroid-induced HPA axis suppression and Cushing's syndrome than mature patients because of a larger skin surface area ...
-
ADVERSE REACTIONS The following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in an ...
-
OVERDOSAGE Topically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects (See - PRECAUTIONS).
-
DOSAGE AND ADMINISTRATIONApply a thin film of Betamethasone Valerate Cream or Ointment to the affected skin areas one to three times a day. Dosage once or twice a day is often effective.
-
HOW SUPPLIEDBetamethasone Valerate Cream USP, 0.1% is supplied as follows: NDC 68071-5224-5 BOX OF 15g - Store at room temperature 15°-30°C (59°-86°F) [see USP Controlled Room Temperature] ...
-
PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information