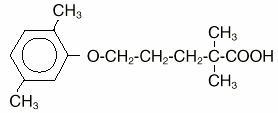
Gemfibrozil is a lipid regulating agent which decreases serum triglycerides and very low density lipoprotein (VLDL) cholesterol, and increases high density lipoprotein (HDL) cholesterol. While ...
Gemfibrozil is a lipid regulating agent which decreases serum triglycerides and very low density lipoprotein (VLDL) cholesterol, and increases high density lipoprotein (HDL) cholesterol. While modest decreases in total and low density lipoprotein (LDL) cholesterol may be observed with gemfibrozil therapy, treatment of patients with elevated triglycerides due to Type IV hyperlipoproteinemia often results in a rise in LDL-cholesterol. LDL-cholesterol levels in Type IIb patients with elevations of both serum LDL-cholesterol and triglycerides are, in general, minimally affected by gemfibrozil treatment; however, gemfibrozil usually raises HDL-cholesterol significantly in this group. Gemfibrozil increases levels of high density lipoprotein (HDL) subfractions HDL2 and HDL3, as well as apolipoproteins AI and AII. Epidemiological studies have shown that both low HDL-cholesterol and high LDL-cholesterol are independent risk factors for coronary heart disease.
In the primary prevention component of the Helsinki Heart Study (refs. 1,2), in which 4081 male patients between the ages of 40 and 55 were studied in a randomized, double-blind, placebo-controlled fashion, gemfibrozil therapy was associated with significant reductions in total plasma triglycerides and a significant increase in high density lipoprotein cholesterol. Moderate reductions in total plasma cholesterol and low density lipoprotein cholesterol were observed for the gemfibrozil treatment group as a whole, but the lipid response was heterogeneous, especially among different Fredrickson types. The study involved subjects with serum non-HDL-cholesterol of over 200 mg/dL and no previous history of coronary heart disease. Over the five-year study period, the gemfibrozil group experienced a 1.4% absolute (34% relative) reduction in the rate of serious coronary events (sudden cardiac deaths plus fatal and nonfatal myocardial infarctions) compared to placebo, p=0.04 (see Table I). There was a 37% relative reduction in the rate of nonfatal myocardial infarction compared to placebo, equivalent to a treatment-related difference of 13.1 events per thousand persons. Deaths from any cause during the double-blind portion of the study totaled 44 (2.2%) in the gemfibrozil randomization group and 43 (2.1%) in the placebo group.
Among Fredrickson types, during the 5-year double-blind portion of the primary prevention component of the Helsinki Heart Study, the greatest reduction in the incidence of serious coronary events occurred in Type IIb patients who had elevations of both LDL-cholesterol and total plasma triglycerides. This subgroup of Type IIb gemfibrozil group patients had a lower mean HDL-cholesterol level at baseline than the Type IIa subgroup that had elevations of LDL-cholesterol and normal plasma triglycerides. The mean increase in HDL-cholesterol among the Type IIb patients in this study was 12.6% compared to placebo. The mean change in LDL-cholesterol among Type IIb patients was –4.1% with gemfibrozil compared to a rise of 3.9% in the placebo subgroup. The Type IIb subjects in the Helsinki Heart Study had 26 fewer coronary events per thousand persons over five years in the gemfibrozil group compared to placebo. The difference in coronary events was substantially greater between gemfibrozil and placebo for that subgroup of patients with the triad of LDL-cholesterol >175 mg/dL (>4.5 mmol), triglycerides >200 mg/dL (>2.2 mmol), and HDL-cholesterol <35 mg/dL (<0.90 mmol) (see Table I).
Further information is available from a 3.5 year (8.5 year cumulative) follow-up of all subjects who had participated in the Helsinki Heart Study. At the completion of the Helsinki Heart Study, subjects could choose to start, stop, or continue to receive gemfibrozil; without knowledge of their own lipid values or double-blind treatment, 60% of patients originally randomized to placebo began therapy with gemfibrozil and 60% of patients originally randomized to gemfibrozil continued medication. After approximately 6.5 years following randomization, all patients were informed of their original treatment group and lipid values during the five years of the double-blind treatment. After further elective changes in gemfibrozil treatment status, 61% of patients in the group originally randomized to gemfibrozil were taking drug; in the group originally randomized to placebo, 65% were taking gemfibrozil. The event rate per 1000 occurring during the open-label follow-up period is detailed in Table II.
Cumulative mortality through 8.5 years showed a 20% relative excess of deaths in the group originally randomized to gemfibrozil versus the originally randomized placebo group and a 20% relative decrease in cardiac events in the group originally randomized to gemfibrozil versus the originally randomized placebo group (see Table III). This analysis of the originally randomized “intent-to-treat’’ population neglects the possible complicating effects of treatment switching during the open-label phase. Adjustment of hazard ratios taking into account open-label treatment status from years 6.5 to 8.5 could change the reported hazard ratios for mortality toward unity.
It is not clear to what extent the findings of the primary prevention component of the Helsinki Heart Study can be extrapolated to other segments of the dyslipidemic population not studied (such as women, younger or older males, or those with lipid abnormalities limited solely to HDL-cholesterol) or to other lipid-altering drugs.
The secondary prevention component of the Helsinki Heart Study was conducted over five years in parallel and at the same centers in Finland in 628 middle-aged males excluded from the primary prevention component of the Helsinki Heart Study because of a history of angina, myocardial infarction or unexplained ECG changes (ref. 3). The primary efficacy endpoint of the study was cardiac events (the sum of fatal and non-fatal myocardial infarctions and sudden cardiac deaths). The hazard ratio (gemfibrozil: placebo) for cardiac events was 1.47 (95% confidence limits 0.88-2.48, p=0.14). Of the 35 patients in the gemfibrozil group who experienced cardiac events, 12 patients suffered events after discontinuation from the study. Of the 24 patients in the placebo group with cardiac events, 4 patients suffered events after discontinuation from the study. There were 17 cardiac deaths in the gemfibrozil group and 8 in the placebo group (hazard ratio 2.18; 95% confidence limits 0.94-5.05, p=0.06). Ten of these deaths in the gemfibrozil group and 3 in the placebo group occurred after discontinuation from therapy. In this study of patients with known or suspected coronary heart disease, no benefit from gemfibrozil treatment was observed in reducing cardiac events or cardiac deaths. Thus, gemfibrozil has shown benefit only in selected dyslipidemic patients without suspected or established coronary heart disease. Even in patients with coronary heart disease and the triad of elevated LDL-cholesterol, elevated triglycerides, plus low HDL-cholesterol, the possible effect of gemfibrozil on coronary events has not been adequately studied.
No efficacy in the patients with established coronary heart disease was observed during the Coronary Drug Project with the chemically and pharmacologically related drug, clofibrate. The Coronary Drug Project was a 6-year randomized, double-blind study involving 1000 clofibrate, 1000 nicotinic acid, and 3000 placebo patients with known coronary heart disease. A clinically and statistically significant reduction in myocardial infarctions was seen in the concurrent nicotinic acid group compared to placebo; no reduction was seen with clofibrate.
The mechanism of action of gemfibrozil has not been definitely established. In man, gemfibrozil has been shown to inhibit peripheral lipolysis and to decrease the hepatic extraction of free fatty acids, thus reducing hepatic triglyceride production. Gemfibrozil inhibits synthesis and increases clearance of VLDL carrier apolipoprotein B, leading to a decrease in VLDL production.
Animal studies suggest that gemfibrozil may, in addition to elevating HDL-cholesterol, reduce incorporation of long-chain fatty acids into newly formed triglycerides, accelerate turnover and removal of cholesterol from the liver, and increase excretion of cholesterol in the feces. Gemfibrozil is well absorbed from the gastrointestinal tract after oral administration. Peak plasma levels occur in 1 to 2 hours with a plasma half-life of 1.5 hours following multiple doses.
Gemfibrozil is completely absorbed after oral administration of gemfibrozil tablets, reaching peak plasma concentrations 1 to 2 hours after dosing. Gemfibrozil pharmacokinetics are affected by the timing of meals relative to time of dosing. In one study (ref. 4), both the rate and extent of absorption of the drug were significantly increased when administered 0.5 hour before meals. Average AUC was reduced by 14-44% when gemfibrozil was administered after meals compared to 0.5 hour before meals. In a subsequent study (ref. 4), rate of absorption of gemfibrozil was maximum when administered 0.5 hour before meals with the Cmax 50-60% greater than when given either with meals or fasting. In this study, there were no significant effects on AUC of timing of dose relative to meals (seeDOSAGE AND ADMINISTRATION).
Gemfibrozil mainly undergoes oxidation of a ring methyl group to successively form a hydroxymethyl and a carboxyl metabolite. Approximately seventy percent of the administered human dose is excreted in the urine, mostly as the glucuronide conjugate, with less than 2% excreted as unchanged gemfibrozil. Six percent of the dose is accounted for in the feces. Gemfibrozil is highly bound to plasma proteins and there is potential for displacement interactions with other drugs (seePRECAUTIONS).
Close