Label: NEFFY- epinephrine spray
- NDC Code(s): 82580-010-02, 82580-020-02
- Packager: ARS Pharmaceuticals Operations, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 17, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NEFFY® safely and effectively. See full prescribing information for NEFFY®. NEFFY® (epinephrine nasal spray) Initial U.S ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGENeffy is indicated for emergency treatment of type I allergic reactions, including anaphylaxis, in adult and pediatric patients aged 4 years and older who weigh 15 kg or greater.
-
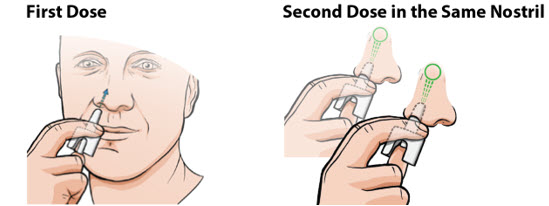
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage for patients aged 4 years and older who weigh 15 kg or greater is based on weight and the dosage is provided in Table 1. For nasal administration ...
-
3 DOSAGE FORMS AND STRENGTHSNasal spray: 1 mg/0.1 mL of epinephrine per spray in a single-dose nasal spray - 2 mg/0.1 mL of epinephrine per spray in a single-dose nasal spray
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Potential Altered Absorption of Neffy in Patients with Underlying Structural or Anatomical Nasal Conditions - Clinical pharmacology studies with neffy included subjects with history of ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Risks Associated with Use of Epinephrine in Certain Coexisting Conditions [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Potential Increased Exposure of Nasal Spray Drugs - Neffy may alter nasal mucosa for up to 2 weeks after administration, and thus may increase systemic absorption of nasal products, including ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Prolonged experience with epinephrine use in pregnant women over several decades, based on published literature, have not identified a drug associated risk of ...
-
10 OVERDOSAGEOverdosage of epinephrine has been reported to produce extremely elevated arterial pressure, which may result in cerebrovascular hemorrhage, particularly in elderly patients. Overdosage may also ...
-
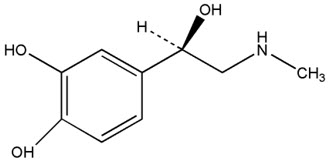
11 DESCRIPTIONNeffy contains epinephrine, a sympathomimetic catecholamine. Chemically, epinephrine is (-)-3,4-dihydroxy-α-[(methylamino)methyl] benzyl alcohol with molecular weight of 183.21 g/mol and the ...
-
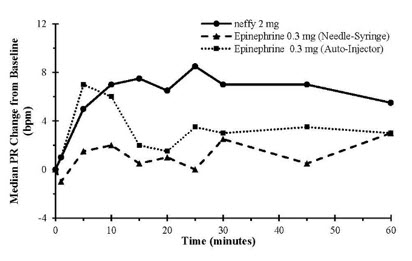
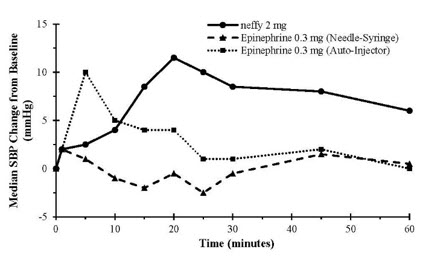
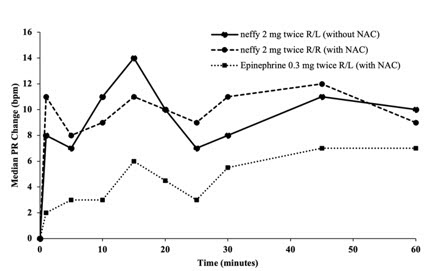
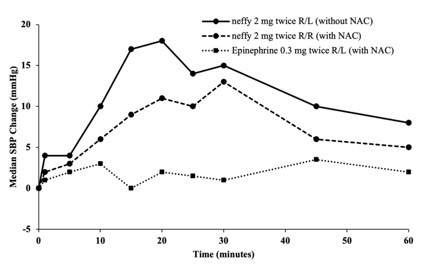
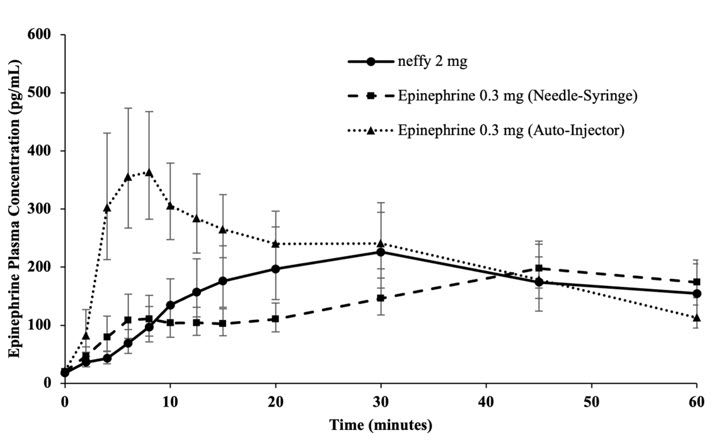
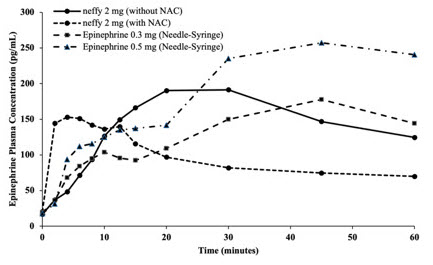
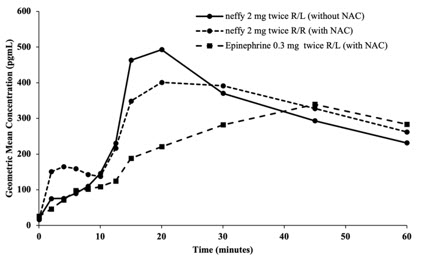
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Epinephrine acts on both alpha and beta-adrenergic receptors. Through its action on alpha-adrenergic receptors, epinephrine lessens the vasodilation and increased ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies to evaluate the carcinogenic potential of epinephrine have not been conducted. Epinephrine and other catecholamines ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Neffy (epinephrine nasal spray) is available in 1 mg and 2 mg strengths as described in Table 4. Table 4: Neffy Strengths and Package Configurations - StrengthPackage ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Administration - Neffy nasal spray is for single use and delivers the entire dose ...
-
SPL UNCLASSIFIED SECTIONManufactured for ARS Pharmaceuticals Operations, Inc., 11682 El Camino Real, San Diego, CA 92130. Neffy is a registered trademark of ARS Pharmaceuticals Operations, Inc. Copyright © 2025 ARS ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - NEFFY® (ne'fee) (epinephrine nasal spray) For allergic emergencies (anaphylaxis) This Patient Information has been approved by the U.S. Food and Drug ...
-
PRINCIPAL DISPLAY PANEL - 2 mg Spray Device Blister Pack CartonDispense in this sealed carton - NDC 82580-020-02 - Rx Only - neffy® (epinephrine nasal spray) 2 mg - For Use in the Nose Only (Single-dose) Read the Instructions for Use for complete - information on how ...
-
PRINCIPAL DISPLAY PANEL - 1 mg Spray Device Blister Pack CartonDispense in this sealed carton - NDC 82580-010-02 - Rx Only - neffy® (epinephrine nasal spray) 1 mg - For Use in the Nose Only (Single-dose) Read the Instructions for Use for complete - information on ...
-
INGREDIENTS AND APPEARANCEProduct Information