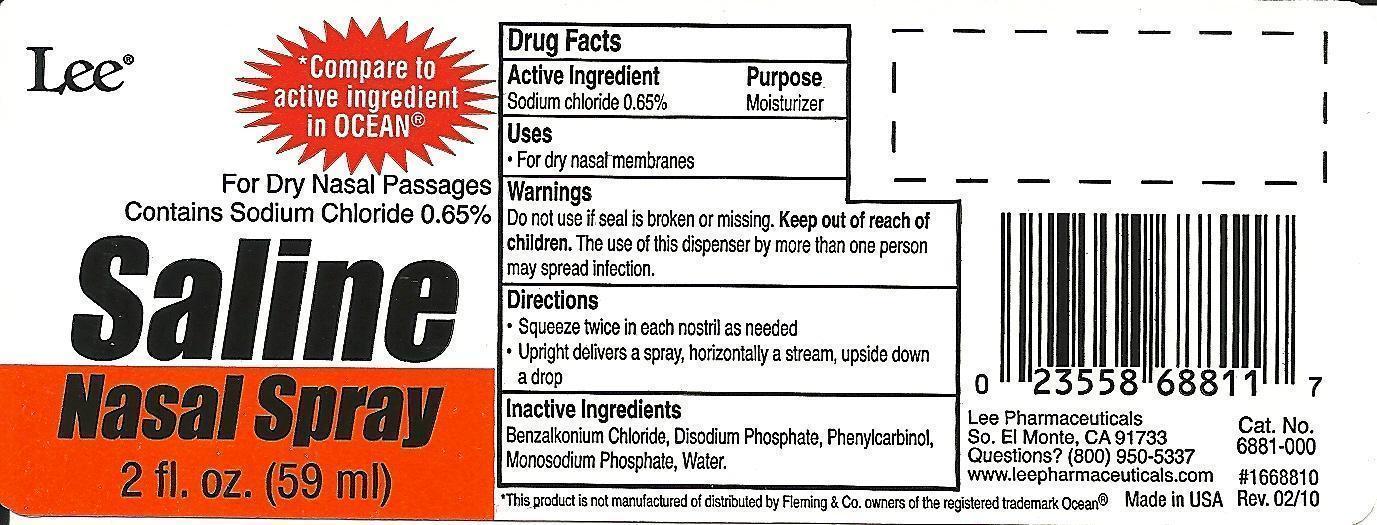
Label: SALINE NASAL 2OZ- sodium chloride 0.65% spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 23558-6881-1 - Packager: Lee Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 15, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient
Sodium Chloride, 0.65%
-
Purpose
Moisturizer
-
Uses
For dry nasal membranes
-
Warnings
Do not use if seal is broken or missing. Keep out of reach of children. The use of this dispenser by more than one person may spread infection.
-
Directions
Squeeze twice in each nostril as needed - Upright delivers a spray, horizontally a stream, upside down a drop
-
Inactive ingredients
Benzalkonium chloride, Disodium phosphate, Phenylcarbinol, Monosodium phosphate, Water
-
PRINCIPAL DISPLAY PANEL
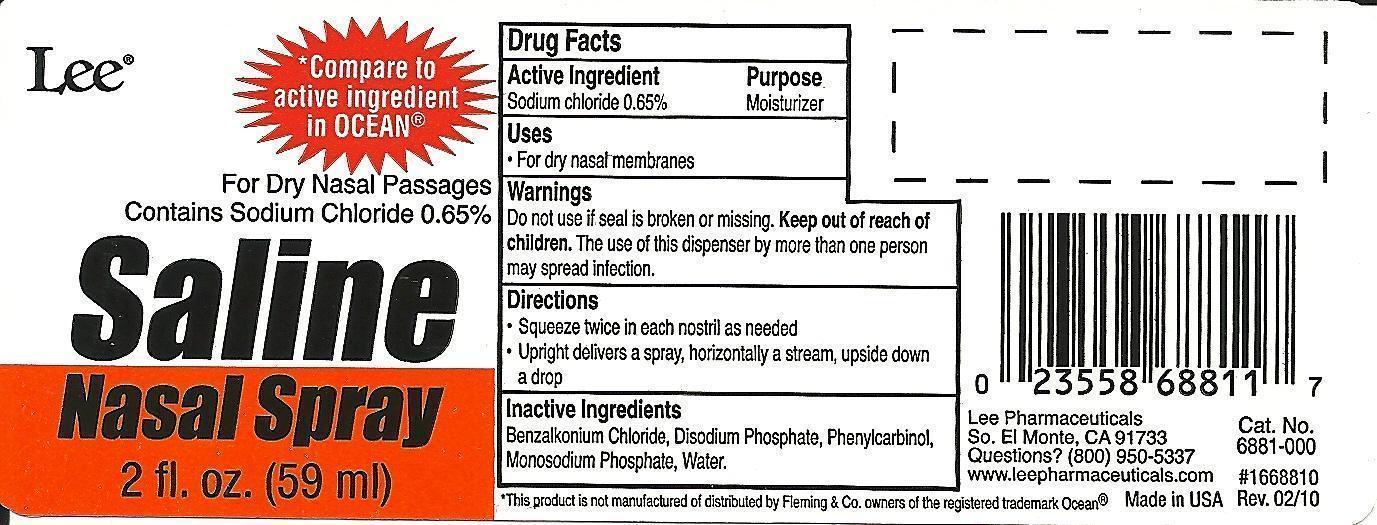
-
INGREDIENTS AND APPEARANCEProduct Information