Label: TASMAR- tolcapone tablet, film coated
- NDC Code(s): 0187-0938-01, 0187-0938-07
- Packager: Bausch Health US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 2, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONBefore prescribing TASMAR®, the physician should be thoroughly familiar with the details of this prescribing information. TASMAR SHOULD NOT BE USED BY PATIENTS UNTIL THERE HAS BEEN A COMPLETE ...
-
BOXED WARNING
(What is this?)
WARNING
Because of the risk of potentially fatal, acute fulminant liver failure, TASMAR (tolcapone) should ordinarily be used in patients with Parkinson's disease on l-dopa/carbidopa who are experiencing symptom fluctuations and are not responding satisfactorily to or are not appropriate candidates for other adjunctive therapies (see INDICATIONS and DOSAGE AND ADMINISTRATION sections).
Because of the risk of liver injury and because TASMAR, when it is effective, provides an observable symptomatic benefit, the patient who fails to show substantial clinical benefit within 3 weeks of initiation of treatment, should be withdrawn from TASMAR.
TASMAR therapy should not be initiated if the patient exhibits clinical evidence of liver disease or two SGPT/ALT or SGOT/AST values greater than the upper limit of normal. Patients with severe dyskinesia or dystonia should be treated with caution (see PRECAUTIONS: Rhabdomyolysis).
PATIENTS WHO DEVELOP EVIDENCE OF HEPATOCELLULAR INJURY WHILE ON TASMAR AND ARE WITHDRAWN FROM THE DRUG FOR ANY REASON MAY BE AT INCREASED RISK FOR LIVER INJURY IF TASMAR IS REINTRODUCED. ACCORDINGLY, SUCH PATIENTS SHOULD NOT ORDINARILY BE CONSIDERED FOR RETREATMENT.
Cases of severe hepatocellular injury, including fulminant liver failure resulting in death, have been reported in postmarketing use. As of May 2005, three cases of fatal fulminant hepatic failure have been reported from more than 40,000 patient years of worldwide use. This incidence may be 10- to 100-fold higher than the background incidence in the general population. Underreporting of cases may lead to significant underestimation of the increased risk associated with the use of TASMAR. All three cases were reported within the first six months of initiation of treatment with TASMAR. Analysis of the laboratory monitoring data in over 3,400 TASMAR-treated patients participating in clinical trials indicated that increases in SGPT/ALT or SGOT/AST, when present, generally occurred within the first 6 months of treatment with TASMAR.
A prescriber who elects to use TASMAR in face of the increased risk of liver injury is strongly advised to monitor patients for evidence of emergent liver injury. Patients should be advised of the need for self-monitoring for both the classical signs of liver disease (e.g., clay colored stools, jaundice) and the nonspecific ones (e.g., fatigue, loss of appetite, lethargy).
Although a program of periodic laboratory monitoring for evidence of hepatocellular injury is recommended, it is not clear that periodic monitoring of liver enzymes will prevent the occurrence of fulminant liver failure. However, it is generally believed that early detection of drug-induced hepatic injury along with immediate withdrawal of the suspect drug enhances the likelihood for recovery. Accordingly, the following liver monitoring program is recommended.
Before starting treatment with TASMAR, the physician should conduct appropriate tests to exclude the presence of liver disease. In patients determined to be appropriate candidates for treatment with TASMAR, serum glutamic-pyruvic transaminase (SGPT/ALT) and serum glutamic-oxaloacetic transaminase (SGOT/AST) levels should be determined at baseline and periodically (i.e., every 2 to 4 weeks) for the first 6 months of therapy. After the first six months, periodic monitoring is recommended at intervals deemed clinically relevant. Although more frequent monitoring increases the chances of early detection, the precise schedule for monitoring is a matter of clinical judgment. If the dose is increased to 200 mg tid (see DOSAGE AND ADMINISTRATION section), liver enzyme monitoring should take place before increasing the dose and then be conducted every 2 to 4 weeks for the following 6 months of therapy. After six months, periodic monitoring is recommended at intervals deemed clinically relevant.
TASMAR should be discontinued if SGPT/ALT or SGOT/AST levels exceed 2 times the upper limit of normal or if clinical signs and symptoms suggest the onset of hepatic dysfunction (persistent nausea, fatigue, lethargy, anorexia, jaundice, dark urine, pruritus, and right upper quadrant tenderness).
Close -
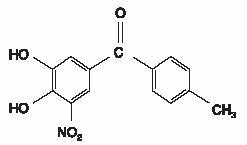
DESCRIPTION:TASMAR is available as tablets containing 100 mg tolcapone. Tolcapone, an inhibitor of catechol-O-methyltransferase (COMT), is used in the treatment of Parkinson's disease as an adjunct to ...
-
CLINICAL PHARMACOLOGY:Mechanism of Action: Tolcapone is a selective and reversible inhibitor of catechol-O-methyltransferase (COMT). In mammals, COMT is distributed throughout various organs. The highest ...
-
INDICATIONS:TASMAR is indicated as an adjunct to levodopa and carbidopa for the treatment of the signs and symptoms of idiopathic Parkinson's disease. Because of the risk of potentially fatal, acute fulminant ...
-
CONTRAINDICATIONS:TASMAR tablets are contraindicated in patients with liver disease, in patients who were withdrawn from TASMAR because of evidence of TASMAR-induced hepatocellular injury or who have demonstrated ...
-
WARNINGS:(see BOXED WARNING) Because of the risk of potentially fatal, acute fulminant liver failure, TASMAR (tolcapone) should ordinarily be used in patients with Parkinson's disease on l-dopa/carbidopa ...
-
PRECAUTIONS:Hypotension/Syncope: Dopaminergic therapy in Parkinson's disease patients has been associated with orthostatic hypotension. Tolcapone enhances levodopa bioavailability and, therefore, may ...
-
Fibrotic Complications:
Cases of retroperitoneal fibrosis, pulmonary infiltrates, pleural effusion, and pleural thickening have been reported in some patients treated with ergot derived dopaminergic agents. While these ...
-
Melanoma:
Epidemiological studies have shown that patients with Parkinson's disease have a higher risk (2- to approximately 6-fold higher) of developing melanoma than the general population. Whether the ...
-
Information for Patients:
Patients should be instructed to take TASMAR only as prescribed. TASMAR should not be used by patients until there has been a complete discussion of the risks and the patient has provided written ...
-
Laboratory Tests:
Although a program of frequent laboratory monitoring for evidence of hepatocellular injury is deemed essential, it is not clear that periodic monitoring of liver enzymes will prevent the ...
-
Special Populations:
TASMAR therapy should not be initiated if the patient exhibits clinical evidence of active liver disease or two SGPT/ALT or SGOT/AST values greater than the upper limit of normal. Patients with ...
-
Drug Interactions:
Protein Binding: Although tolcapone is highly protein bound, in vitro studies have shown that tolcapone at a concentration of 50 mcg/mL did not displace other highly protein-bound drugs from ...
-
Carcinogenesis, Mutagenesis and Impairment of Fertility:
Carcinogenesis: Carcinogenicity studies in which tolcapone was administered in the diet were conducted in mice and rats. Mice were treated for 80 (female) or 95 (male) weeks with doses of ...
-
Pregnancy:Tolcapone, when administered alone during organogenesis, was not teratogenic at doses of up to 300 mg/kg/day in rats or up to 400 mg/kg/day in rabbits (5.7 times and 15 times the recommended daily ...
-
ADVERSE REACTIONS:Cases of severe hepatocellular injury, including fulminant liver failure resulting in death, have been reported in postmarketing use. As of May 2005, three cases of fatal fulminant hepatic ...
-
DRUG ABUSE AND DEPENDENCE:Tolcapone is not a controlled substance. Studies conducted in rats and monkeys did not reveal any potential for physical or psychological dependence. Although clinical trials have not revealed any ...
-
OVERDOSAGE:The highest dose of tolcapone administered to humans was 800 mg tid, with and without levodopa/carbidopa co-administration. This was in a 1-week study in elderly, healthy volunteers. The peak ...
-
DOSAGE AND ADMINISTRATION:Because of the risk of potentially fatal, acute fulminant liver failure, TASMAR (tolcapone) should ordinarily be used in patients with Parkinson's disease on l-dopa/carbidopa who are experiencing ...
-
HOW SUPPLIED:TASMAR is supplied as film-coated tablets containing 100 mg tolcapone. The 100 mg beige to yellowish beige tablet is hexagonal and biconvex. Debossed on one side of the 100 mg tablet is TASMAR ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Bausch Health US, LLC - Bridgewater, NJ 08807 USA - Manufactured by: Bausch Health Companies Inc. Steinbach, MB R5G 1Z7, Canada - TASMAR is a trademark of Bausch Health Companies ...
-
SPL UNCLASSIFIED SECTIONPATIENT ACKNOWLEDGEMENT OF RISKS - ASSOCIATED WITH TASMAR TREATMENT - The following is important information that patients should know about TASMAR. • TASMAR should not be used ...
-
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle LabelNDC 0187-0938-01 - Rx Only - Tasmar ® (tolcapone) Tablets - 90 Tablets - 100 mg - Each tablet contains - 100 mg tolcapone - BAUSCH Health
-
INGREDIENTS AND APPEARANCEProduct Information