Label: PURIXAN suspension
- NDC Code(s): 62484-0020-1, 62484-0020-2
- Packager: Nova Laboratories, Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PURIXAN safely and effectively. See full prescribing information for PURIXAN.
PURIXAN(R) (mercaptopurine) oral suspension
Initial U.S. Approval: 1953RECENT MAJOR CHANGES
Warnings and Precautions, Hepatotoxicity (5.2) 7/2024 INDICATIONS AND USAGE
PURIXAN is a nucleoside metabolic inhibitor indicated for the treatment of patients with acute lymphoblastic leukemia (ALL) as part of a combination chemotherapy maintenance regimen.(1.1)
DOSAGE AND ADMINISTRATION
- The recommended starting dosage of PURIXAN is 1.5 mg/kg to 2.5 mg/kg (50 mg/m2 to 75 mg/m2) orally once daily as part of a combination chemotherapy maintenance regimen. Adjust dose to maintain desirable absolute neutrophil count and for excessive myelosuppression.(2.1)
- Renal Impairment: Use the lowest recommended starting dose or increase the dosing interval. (2.3,8.6)
- Hepatic Impairment: Use the lowest recommended starting dose. (2.3,8.7)
DOSAGE FORMS AND STRENGTHS
Oral suspension: 2,000 mg/100 mL (20 mg/mL). (3)
CONTRAINDICATIONS
- None
WARNINGS AND PRECAUTIONS
- Myelosuppression: Monitor complete blood count (CBC) and adjust the dose of PURIXAN for excessive myelosuppression. Consider testing in patients with severe myelosuppression or repeated episodes of myelosuppression for thiopurine S-methyltransferase (TPMT) or nucleotide diphosphatase (NUDT15) deficiency. Patients with homozygous-TPMT or homozygous-NUDT15 deficiency may require a dose reduction. (2.2,5.1)
- Hepatotoxicity: Monitor transaminases, alkaline phosphatase and bilirubin. Withhold PURIXAN at onset of hepatotoxicity. (5.2)
- Immunosuppression:Response to all vaccines may be diminished and there is a risk of infection with live virus vaccines. Consult immunization guidelines for immunocompromised pediatrics. (5.3)
- Treatment Related Malignancies: Aggressive and fatal cases of hepatosplenic T-cell lymphoma have occurred. (5.4)
- Macrophage Activation Syndrome: Monitor for and treat promptly; discontinue PURIXAN. (5.5)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise patients of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.6, 8.1, 8.3)
ADVERSE REACTIONS
The most common adverse reaction (> 20%) is myelosuppression including anemia, neutropenia, lymphopenia and thrombocytopenia. Adverse reactions occurring in 5% to 20% of patients include anorexia, nausea, vomiting, diarrhea, malaise and rash. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Rare Disease Therapeutics, Inc., at 1-844-472-7389 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Acute Lymphoblastic Leukemia
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Modifications in Patients with TPMT and/or NUDT15 Deficiency
2.3 Dosage Modifications in Renal and Hepatic Impairment
2.4 Dosage Modification with Concomitant Use of Allopurinol
2.5 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
5.2 Hepatotoxicity
5.3 Immunosuppression
5.4 Treatment Related Malignancies
5.5 Macrophage Activation Syndrome
5.6 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Allopurinol
7.2 Warfarin
7.3 Myelosuppressive Products
7.4 Aminosalicylates
7.5 Hepatotoxic Products
7.6 Methotrexate
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended starting dose of PURIXAN is 1.5 mg/kg to 2.5 mg/kg (50 mg/m2 to 75 mg/m2) orally once daily as part of combination chemotherapy maintenance regimen. Take PURIXAN either consistently with or without food.
After initiating PURIXAN, monitor complete blood counts (CBC) and adjust the dose to maintain absolute neutrophil count (ANC) at a desirable level and for excessive myelosuppression. Evaluate the bone marrow in patients with prolonged myelosuppression or repeated episodes of myelosuppression to assess leukemia status and marrow cellularity.
Evaluate thiopurine S-methyltransferase (TPMT) and nucleotide diphosphatase (NUDT15) status in patients with severe myelosuppression or repeated episodes of myelosuppression [see Dosage and Administration (2.2)].
If a patient misses a dose, instruct the patient to continue with the next scheduled dose.2.2 Dosage Modifications in Patients with TPMT and/or NUDT15 Deficiency
Consider testing for TPMT and NUDT15 deficiency in patients who experience severe myelosuppression or repeated episodes of myelosuppression [see Warnings and Precautions (5.1)and Clinical Pharmacology (12.5)].
Homozygous Deficiency in either TPMT or NUDT15
Patients with homozygous deficiency of either enzyme typically require 10% or less of the recommended dosage. Reduce the recommended starting dosage of PURIXAN in patients who are known to have homozygous TPMT or NUDT15 deficiency.
Heterozygous Deficiency in TPMT and/or NUDT15
Reduce the PURIXAN dosage based on tolerability. Most patients with heterozygous TPMT or NUDT15 deficiency tolerate recommended dosage, but some require dose reduction based on adverse reactions. Patients who are heterozygous for both TPMT and NUDT15 may require more substantial dose reductions.2.3 Dosage Modifications in Renal and Hepatic Impairment
Renal Impairment
Use the lowest recommended starting dosage for PURIXAN in patients with renal impairment (CLcr less than 50 mL/min). Adjust the dosage to maintain absolute neutrophil count (ANC) at a desirable level and for adverse reactions [see Uses in Specific Populations (8.6)].
Hepatic Impairment
Use the lowest recommended starting dosage for PURIXAN in patients with hepatic impairment. Adjust the dosage to maintain absolute neutrophil count (ANC) at a desirable level and for adverse reactions [see Uses in Specific Populations (8.7)].
2.4 Dosage Modification with Concomitant Use of Allopurinol
Reduce the dose of PURIXAN to one-third to one-quarter of the current dosage when coadministered with allopurinol [see Drug Interactions (7.1)].
2.5 Administration
Shake the bottle vigorously for at least 30 seconds to ensure the oral suspension is well mixed. PURIXAN is a pink to brown viscous oral suspension.
Provide a press-in bottle adapter and two oral dispensing syringes (one 1 mL and one 5 mL).
Train patients or caregivers on proper handling, storage, administration, disposal and clean-up of accidental spillage prior to initiation of PURIXAN and during each visit to the clinic.
Advise patients and caregivers to use PURIXAN within 8 weeks and properly discard remaining PURIXAN after 8 weeks.
Provide instructions regarding which syringe to use and how to administer the specified dose, since PURIXAN is supplied with 1 mL and 5 mL oral dispensing syringes.
Advise patients that the oral dispensing syringe is intended for multiple uses and provide the following instructions:- Wash the oral dispensing syringe with warm ‘soapy’ water and rinse well;
- Hold the oral dispensing syringe under water and move the plunger up and down several times to make sure the inside of the oral dispensing syringe is clean;
- Ensure the oral dispensing syringe is completely dry before use of the oral dispensing syringe again; and
- Store the oral dispensing syringe in a hygienic place with PURIXAN.
PURIXAN is a hazardous drug. Follow special handling and disposal procedures.1
5.1 Myelosuppression
The most consistent, dose-related adverse reaction of PURIXAN is myelosuppression, manifested by anemia, leukopenia, thrombocytopenia, or any combination of these. Monitor CBC and adjust the dosage of PURIXAN for excessive myelosuppression [see Dosage and Administration (2.1)].
Consider testing for thiopurine S-methyltransferase (TPMT) or nucleotide diphosphatase (NUDT15) deficiency in patients with severe myelosuppression or repeated episodes of myelosuppression. TPMT genotyping or phenotyping (red blood cell TPMT activity) and NUDT15 genotyping can identify patients who have reduced activity of these enzymes. Patients with homozygous TPMT or NUDT15 deficiency may require a dose reduction, [see Dosage and Administration (2.2), Clinical Pharmacology (12.5)].
Myelosuppression can be exacerbated by coadministration with allopurinol, aminosalicylates or other products that cause myelosuppression. [see Drug Interactions (7.1, 7.3 and 7.4)]. Reduce the dosage of PURIXAN when coadministered with allopurinol [see Dosage and Administration (2.4)].5.2 Hepatotoxicity
Mercaptopurine is hepatotoxic. There are reports of deaths attributed to hepatic necrosis associated with the administration of mercaptopurine. Hepatic injury can occur with any dosage but seems to occur with greater frequency when the recommended dosage is exceeded. In some patients, jaundice has cleared following withdrawal of mercaptopurine and reappeared with rechallenge.
Usually, clinically detectable jaundice appears early in the course of treatment (1 to 2 months); however, jaundice has been reported as early as 1 week and as late as 8 years after starting mercaptopurine. The hepatotoxicity has been associated in some cases with anorexia, diarrhea, jaundice, ascites and pruritus. Hepatic encephalopathy has occurred.
Monitor serum transaminase levels, alkaline phosphatase, and bilirubin levels at weekly intervals when first beginning therapy and at monthly intervals thereafter. Monitor liver tests more frequently in patients who are receiving PURIXAN with other hepatotoxic drugs [see Drug Interactions (7.5)] or with known pre-existing liver disease. Withhold PURIXAN at onset of hepatotoxicity.
Intrahepatic Cholestasis of Pregnancy
Postmarketing cases of intrahepatic cholestasis of pregnancy (ICP) have been reported in patients with inflammatory bowel disease who received mercaptopurine during pregnancy. PURIXAN is not indicated for use in inflammatory bowel disease [see Indications and Usage (1.1)].
Discontinue PURIXAN if ICP develops in a pregnant woman.5.3 Immunosuppression
Mercaptopurine is immunosuppressive and may impair the immune response to infectious agents or vaccines. Due to the immunosuppression associated with maintenance chemotherapy for ALL, response to all vaccines may be diminished and there is a risk of infection with live virus vaccines. Consult immunization guidelines for immunocompromised patients.
5.4 Treatment Related Malignancies
Hepatosplenic T-cell lymphoma has been reported in patients treated with mercaptopurine for inflammatory bowel disease (IBD), an unapproved use. Mercaptopurine is mutagenic in animals and humans, carcinogenic in animals, and may increase the risk of secondary malignancies.
Patients receiving immunosuppressive therapy, including mercaptopurine, are at an increased risk of developing lymphoproliferative disorders and other malignancies, notably skin cancers (melanoma and non-melanoma), sarcomas (Kaposi's and non-Kaposi's) and uterine cervical cancer in situ. The increased risk appears to be related to the degree and duration of immunosuppression. It has been reported that discontinuation of immunosuppression may provide partial regression of the lymphoproliferative disorder.
A treatment regimen containing multiple immunosuppressants (including thiopurines) should therefore be used with caution as this could lead to lymphoproliferative disorders, some with reported fatalities. A combination of multiple immunosuppressants, given concomitantly increases the risk of Epstein-Barr virus (EBV)-associated lymphoproliferative disorders.5.5 Macrophage Activation Syndrome
Macrophage activation syndrome (MAS) (hemophagocytic lymphohistiocytosis) is a known, life-threatening disorder that may develop in patients with autoimmune conditions, in particular with inflammatory bowel disease (IBD), and there could potentially be an increased susceptibility for developing the condition with the use of mercaptopurine (an unapproved use). If MAS occurs, or is suspected, discontinue PURIXAN. Monitor for and promptly treat infections such as EBV and cytomegalovirus (CMV), as these are known triggers for MAS.
5.6 Embryo-Fetal Toxicity
PURIXAN can cause fetal harm when administered to a pregnant woman. An increased incidence of miscarriage has been reported in women who received mercaptopurine in the first trimester of pregnancy. Adverse embryo-fetal findings, including miscarriage and stillbirth, have been reported in women who received mercaptopurine after the first trimester of pregnancy. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with PURIXAN and for 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with PURIXAN and for 3 months after the last dose [see Use in Specific Populations (8.1,8.3)].
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Myelosuppression [see Warnings and Precautions (5.1)]
- Hepatotoxicity [see Warnings and Precautions (5.2)]
- Immunosuppression [see Warnings and Precautions (5.3)]
- Treatment Related Malignancies [see Warnings and Precautions (5.4)]
- Macrophage Activation Syndrome [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Based on multicenter cooperative group ALL trials, the most common adverse reaction occurring in > 20% of patients was mylelosuppression including anemia, neutropenia, lymphopenia and thrombocytopenia. Adverse reactions occurring in 5% to 20% of patients included anorexia, nausea, vomiting, diarrhea, malaise, and rash. Adverse reactions occurring in < 5 % of patients included urticaria, hyperuricemia, oral lesions, elevated transaminases, hyperbilirubinemia, hyperpigmentation, infections, and pancreatitis. Oral lesions resemble thrush rather than antifolic ulcerations. Delayed or late toxicities include hepatic fibrosis, hyperbilirubinemia, alopecia, pulmonary fibrosis, oligospermia and secondary malignancies. [see Warnings and Precautions (5.1, 5.2)].
Drug fever has been reported with PURIXAN.6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of PURIXAN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reactions include: photosensitivity, hypoglycemia, portal hypertension, intrahepatic cholestasis of pregnancy (ICP), pellagra, and erythema nodosum.
7.1 Allopurinol
Allopurinol can inhibit the first-pass oxidative metabolism of mercaptopurine by xanthine oxidase, which can lead to an increased risk of mercaptopurine adverse reactions [see Warnings and Precautions (5.1), Adverse Reactions (6.1)]. Reduce the dose of PURIXAN when coadministered with allopurinol [see Dosage and Administration (2.4)].
7.2 Warfarin
The coadministration of PURIXAN with warfarin may decrease the anticoagulant effectiveness of warfarin. Monitor the international normalized ratio (INR) in patients receiving warfarin and adjust the warfarin dosage as appropriate.
7.3 Myelosuppressive Products
PURIXAN can cause myelosuppression. Myelosuppression may be increased when PURIXAN is coadministered with other drugs that cause myelosuppression. Enhanced myelosuppression has been noted in some patients receiving trimethoprim-sulfamethoxazole. Monitor the CBC and adjust the dose of PURIXAN for excessive myelosuppression [see Dosage and Administration (2.1), Warnings and Precautions (5.1)].
7.4 Aminosalicylates
Aminosalicylates (e.g., mesalamine, olsalazine or sulfasalazine) may inhibit the TPMT enzyme, which may increase the risk of myelosuppression when coadministered with PURIXAN. When aminosalicylates and PURIXAN are coadministered, use the lowest possible doses for each drug and monitor more frequently for myelosuppression [see Warnings and Precautions (5.1)].
7.5 Hepatotoxic Products
PURIXAN can cause hepatotoxicity. Hepatotoxicity may be increased when PURIXAN is coadministered with other products that cause hepatotoxicity. Monitor liver tests more frequently in patients who are receiving PURIXAN with other hepatotoxic products [see Warnings and Precautions (5.2)].
7.6 Methotrexate
PURIXAN dosage may need adjustment when administered concomitantly with high dose methotrexate [see Warnings and Precautions (5.1)]. Mercaptopurine exposure increases with concomitant methotrexate use [see Clinical Pharmacology (12.3)] which may increase the risk of PURIXAN adverse reactions. The mechanism of this interaction has not been fully characterized [see Clinical Pharmacology (12.3)].
8.1 Pregnancy
Risk Summary
PURIXAN can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. Pregnant women who receive mercaptopurine have an increased incidence of miscarriage and stillbirth (see Data). Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
Women receiving mercaptopurine in the first trimester of pregnancy have an increased incidence of miscarriage; the risk of malformation in offspring surviving first trimester exposure is not known. In a series of 28 women receiving mercaptopurine after the first trimester of pregnancy, 3 mothers died prior to delivery, 1 delivered a stillborn child, and 1 aborted; there were no cases of macroscopically abnormal fetuses.
Animal Data
Mercaptopurine was embryo-lethal and teratogenic in several animal species (rat, mouse, rabbit, and hamster) at doses less than the recommended human dose.8.2 Lactation
Risk Summary
There are no data on the presence of mercaptopurine or its metabolites in human milk, the effects on the breastfed child or the effects on milk production. Because of the potential for serious adverse reactions in the breastfed child, advise women not to breastfeed during treatment with PURIXAN and for 1 week after the last dose.8.3 Females and Males of Reproductive Potential
PURIXAN can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify the pregnancy status in females of reproductive potential prior to initiating PURIXAN [see Use in Specific Populations (8.1)].
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with PURIXAN and for 6 months after the last dose.
Males
Based on genotoxicity findings, advise males with female partners of reproductive potential to use effective contraception during treatment with PURIXAN and for 3 months after the last dose [see Nonclinical Toxicology (13.1)].
Infertility
Females and Males
Based on findings from animal studies, PURIXAN can impair female and male fertility [see Nonclinical Toxicology (13.1)]. The long-term effects of mercaptopurine on female and male fertility, including the reversibility have not been studied.8.4 Pediatric Use
Safety and effectiveness of PURIXAN has been established in pediatric patients. Use of PURIXAN in pediatrics is supported by evidence from the published literature and clinical experience. Symptomatic hypoglycemia has been reported in pediatric patients with ALL receiving mercaptopurine. Reported cases were in pediatrics less than 6 years or with a low body mass index.
8.5 Geriatric Use
Clinical studies of mercaptopurine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or another drug therapy.
8.6 Renal Impairment
Use the lowest recommended starting dosage for PURIXAN or increase the dosing interval to every 36 to 48 hours in patients with renal impairment (CLcr less than 50 mL/min). Adjust the dose to maintain absolute neutrophil count (ANC) at a desirable level and for adverse reactions [see Dosage and Administration (2.3)].
8.7 Hepatic Impairment
Use the lowest recommended starting dosage for PURIXAN in patients with hepatic impairment. Adjust the dose to maintain absolute neutrophil count (ANC) at a desirable level and for adverse reactions [see Dosage and Administration (2.3)].
-
10 OVERDOSAGE
Signs and symptoms of mercaptopurine overdosage may be immediate (anorexia, nausea, vomiting, and diarrhea) or delayed (myelosuppression, liver dysfunction, and gastroenteritis). Dialysis cannot be expected to clear mercaptopurine. Hemodialysis is thought to be of marginal use due to the rapid intracellular incorporation of mercaptopurine into active metabolites with long persistence.
Withhold PURIXAN immediately if severe or life-threatening adverse reactions occur during treatment. If a patient is seen immediately following an accidental overdosage, it may be useful to induce emesis. -
11 DESCRIPTION
Mercaptopurine, a nucleoside metabolic inhibitor. The chemical name is 1,7-dihydro-6H-purine-6-thione monohydrate. The molecular formula is C5H4N4S•H2O and the molecular weight is 170.20. The structural formula is:
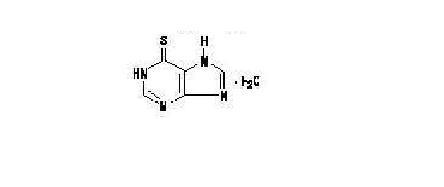
Mercaptopurine is a yellow, odorless or practically odorless, crystalline powder. It is practically insoluble in water with pKa 7.8, 11.2.
PURIXAN (mercaptopurine) oral suspension contains 2,000 mg/100 mL (20 mg/mL) of mercaptopurine. The suspension also contains the following inactive ingredients: xanthan gum, aspartame, concentrated raspberry juice, sucrose, ethyl parahydroxybenzoate sodium, methyl parahydroxybenzoate sodium, potassium sorbate, sodium hydroxide and purified water. PURIXAN is a pink to brown viscous suspension. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mercaptopurine is a purine analog that undergoes intracellular transport and activation to form metabolites including thioguanine nucleotides (TGNs). Incorporation of TGNs into DNA or RNA results in cell-cycle arrest and cell death. TGNs and other mercaptopurine metabolites are also inhibitors of de novo purine synthesis and purine nucleotide interconversions. Mercaptopurine was cytotoxic to proliferating cancer cells in vitro and had antitumor activity in mouse tumor models. It is not known which of the biochemical effects of mercaptopurine and its metabolites are directly or predominantly responsible for cell death.
12.2 Pharmacodynamics
Exposure-Response Relationships
Mercaptopurine exposure-response relationships and the time course of pharmacodynamics response are unknown.12.3 Pharmacokinetics
Following a single oral dose of PURIXAN 50 mg under fasted conditions to adult healthy subjects, the median (min – max) AUC0-INF was 137 h∙ng/mL (77 – 268 h∙ng/mL) and Cmax was 93 ng/mL (40 – 204 ng/mL).
Absorption
PURIXAN is absorbed after oral administration with a median (min – max) Tmax of 0.75 (0.33 – 2.5) hours.
Effect of foods
Food has been shown to decrease the exposure of mercaptopurine.
Distribution
The volume of distribution exceeds total body water with a mean volume of distribution of approximately 0.9 L/kg. There is negligible entry of mercaptopurine into cerebrospinal fluid.
Plasma protein binding averages 19% over the concentration range 10 to 50 mcg/L.
Elimination
The median (min – max) elimination half-life (t1/2) was 1.3 (0.9 – 5.4) hours.
Metabolism
Mercaptopurine is inactivated via two major pathways. One is thiol methylation, which is catalyzed by the polymorphic enzyme thiopurine S-methyltransferase (TPMT), to form the inactive metabolite methyl-mercaptopurine. The second inactivation pathway is oxidation, which is catalyzed by xanthine oxidase. The product of oxidation is the inactive metabolite 6-thiouric acid.
Elimination
After oral administration of mercaptopurine, urine contains intact mercaptopurine, thiouric acid (formed by direct oxidation by xanthine oxidase, probably via 6-mercapto-8-hydroxypurine), and a number of 6-methylated thiopurines. In one subject, a total of 46% of the dose could be accounted for in the urine (as parent drug and metabolites) in the first 24 hours.
Specific Populations
Pediatrics Patients
Wide inter individual variations in systemic exposure is observed. Following oral administration of 50 mg/m2 mercaptopurine in 10 children, median plasma concentrations 1 hour post dose was 0.35 (range 0.03 to 1.03) μM and median AUC1–5hours was 56 (range 23 to 65) μM·min. Tmax ranged from 1 to 3 hours.
Drug Interactions Studies
Methotrexate: Mercaptopurine AUC increased by approximately 31% when coadministered with oral methotrexate 20 mg/m2. Mercaptopurine AUC increased by 69% when coadministered with IV methotrexate 2 g/m2, and by 93% when coadministered with IV methotrexate 5 g/m2.12.5 Pharmacogenomics
Several published studies indicate that patients with reduced TPMT or NUDT15 activity receiving usual doses of mercaptopurine, accumulate excessive cellular concentrations of active 6-TGNs, and are at higher risk for severe myelosuppression [see Warnings and Precautions (5.1)]. In a study of 1028 children with ALL, the approximate tolerated mercaptopurine dosage for patients with TPMT and/or NUDT15 deficiency on mercaptopurine maintenance therapy (as a percentage of the planned dosage) was as follows: heterozygous for either TPMT or NUDT15, 50-90%; heterozygous for both TPMT and NUDT15, 30-50%; homozygous for either TPMT or NUDT15, 5-10%.
Approximately 0.3% (1:300) of patients of European or African ancestry have two loss-of-function alleles of the TPMT gene and have little or no TPMT activity (homozygous deficient or poor metabolizers), and approximately 10% of patients have one loss-of-function TPMT allele leading to intermediate TPMT activity (heterozygous deficient or intermediate metabolizers). The TPMT*2, TPMT*3A, and TPMT*3C alleles account for about 95% of individuals with reduced levels of TPMT activity.
NUDT15 deficiency is detected in <1% of patients of European or African ancestry. Among patients of East Asian ancestry (i.e., Chinese, Japanese, Vietnamese), 2% have two loss-of-function alleles of the NUDT15 gene, and approximately 21% have one loss-of-function allele. The p.R139C variant of NUDT15 (present on the *2 and *3 alleles) is the most commonly observed, but other less common loss-of-function NUDT15 alleles have been observed.
Consider all clinical information when interpreting results from phenotypic testing used to determine the level of thiopurine nucleotides or TPMT activity in erythrocytes, since some coadministered drugs can influence measurement of TPMT activity in blood, and blood from recent transfusions will misrepresent a patient’s actual TPMT activity [see Dosage and Administration (2.2) and Warnings and Precautions (5.1)]. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Mercaptopurine is carcinogenic in animals.
Mercaptopurine causes chromosomal aberrations in cells derived from animals and humans and induces dominant-lethal mutations in the germs cells of male mice.
Mercaptopurine can impair fertility. In mice, surviving female offspring of mothers who received chronic low doses of mercaptopurine during pregnancy were found sterile, or if they became pregnant, had smaller litters and more dead fetuses as compared to control animals. - 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
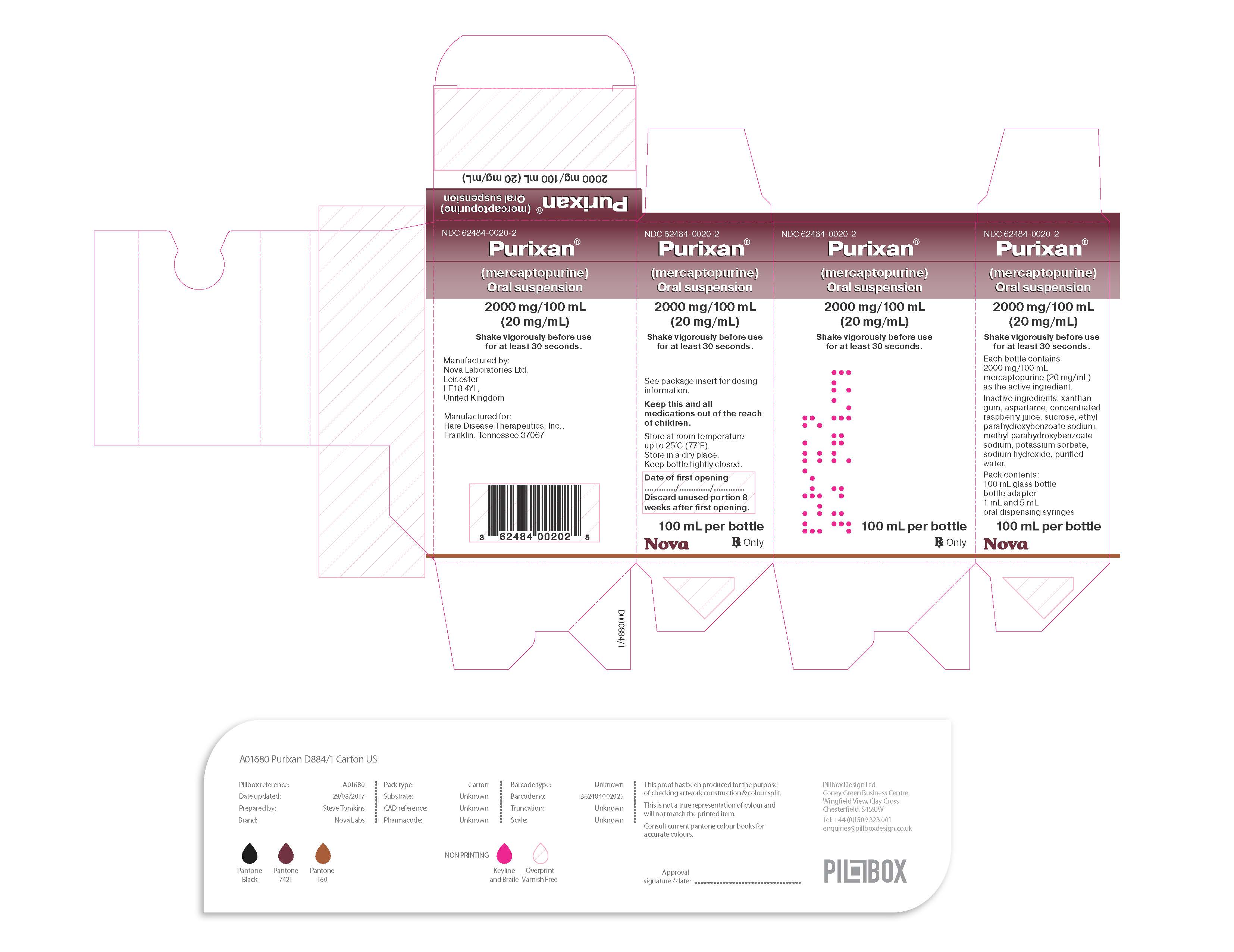
PURIXAN (mercaptopurine) oral suspension is supply as 2,000 mg/100 mL (20 mg/mL) is a pink to brown viscous liquid in amber glass multiple-dose bottles. In addition, a press-in bottle adapter and two oral dispensing syringes (one 1 mL and one 5 mL) are provided.
Each carton NDC 62484-0020-2 contains 1 bottle of PURIXAN NDC 62484-0020-1.- Store PURIXAN between 59ºF to 77ºF (15ºC to 25ºC). Store in a dry place.
PURIXAN is a hazardous drug. Follow special handling and disposal procedures.1
-
17 PATIENT COUNSELING INFORMATION
Advise the patients and caregivers to read the FDA-approved patient labelling (Patient Information and Instructions for Use).
Major Adverse Events
Advice patients and caregivers that PURIXAN can cause myelosuppression, hepatotoxicity, and gastrointestinal toxicity. Advise patients to contact their healthcare provider if they experience fever, sore throat, jaundice, nausea, vomiting, signs of local infection, bleeding from any site, or symptoms suggestive of anemia [see Warnings and Precautions (5.1, 5.2, 5.3)].
Proper Preparation and Administration
Advise patients or caregivers on proper handling, storage, preparation, administration, and disposal and clean-up of accidental spillage of the medication prior to initiation and on each visit to the clinic [see Dosage and Administration (2.5)].
Embryo-Fetal Toxicity- Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.2, 5.6), Use in Specific Populations (8.1)].
- Advise females of reproductive potential to use effective contraception during treatment with PURIXAN and for 6 months after the last dose [see Use in Specific Populations (8.3)].
- Advise males with female partners of reproductive potential to use effective contraception during treatment with PURIXAN and for 3 months after the last dose [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Lactation
Advise women not to breastfeed during treatment with PURIXAN and for 1 week after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise males and females of reproductive potential that PURIXAN can impair fertility [see Use in Specific Populations (8.3)].
Other Adverse Reactions
Instruct patients to minimize sun exposure due to risk of photosensitivity [see Adverse Reactions (6.2)].
Manufactured by:
Nova Laboratories Ltd
Leicester
LE 18 4YL
United KingdomManufactured for:
Rare Disease Therapeutics
2550 Meridian Blvd., Suite 150
Franklin, TN 37067
www.raretx.com
Part Number: D001355/1
-
PATIENT MEDICATION INFORMATION
PATIENT INFORMATION
PURIXAN®(pure-ee-zan)
(mercaptopurine)
oral suspensionWhat is PURIXAN?
PURIXAN is a prescription medicine used along with other medicines to treat people with acute lymphoblastic leukemia (ALL).What should I tell my healthcare provider before taking PURIXAN?
• have kidney or liver problems.
Before you take PURIXAN, tell your healthcare provider about all of your medical conditions,including if you:
• have a condition where your body produces too little of the enzyme thiopurine methyltransferase (TPMT) or the enzyme nucleotide diphosphatase (NUDT15).
• have recently received or plan to receive a vaccine.
• are pregnant or plan to become pregnant. PURIXAN can harm your unborn baby.
If you are a female who is able to become pregnant:o Your healthcare provider will do a pregnancy test before you start treatment with PURIXAN.
If you are a male with a female partner who is able to become pregnant:
o Use an effective method of birth control (contraception) during treatment with PURIXAN and for 6 months after your last dose. Talk with your healthcare provider about birth control methods you can
use during this time.
o Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with PURIXAN.
o Use effective birth control (contraception) during treatment with PURIXAN and for 3 months after your last dose.
o Tell your healthcare provider right away if your female partner becomes pregnant or thinks she is pregnant during your treatment with PURIXAN.- are breastfeeding or plan to breastfeed. Do not breastfeed during treatment with PURIXAN and for at least 1 week after your last dose.
- Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take PURIXAN? - See the detailed “Instructions for Use” that comes with PURIXAN for information about the right way to measure and take a dose of PURIXAN.
- Take PURIXAN exactly as your healthcare provider tells you. Do not stop taking PURIXAN or change your dose without talking to your healthcare provider.
- Take PURIXAN by mouth 1 time each day.
-
If PURIXAN comes into contact with skin, eyes, or clothes?
• Remove contaminated clothing.
• Wash skin or eyes immediately with water.
• Contact with skin or eyes can cause hypersensitive reactions resulting in rash, redness, itching and inflammation. If symptoms appear seek medical attention. - During treatment with PURIXAN, your healthcare provider will do blood tests regularly to check your blood cell counts and liver function. Your healthcare provider may change your dose if you have side effects.
- If you miss a dose of PURIXAN, call your healthcare provider for advice.
- If you take too much PURIXAN, call your healthcare provider or go to the nearest emergency room right away.
What should I avoid while taking PURIXAN?
PURIXAN can make your skin more sensitive to sunlight. Protect yourself from sunlight during treatment with PURIXAN.What are the possible side effects of PURIXAN?
PURIXAN can cause serious side effects, including:-
Decreased blood cell countsare common with PURIXAN, but can also be severe. PURIXAN affects your bone marrow and can cause decreased white blood cells, red blood cells, and platelets.
Decreased blood cell counts can make you more likely to develop infections, bleeding, or anemia. If you take certain medicines during treatment with PURIXAN, it could make the effects on your bone marrow worse.
Tell your healthcare provider if you develop any of the following symptoms during treatment with PURIXAN:
• fever
• sore throat
• cuts or wounds that are red, or swollen, or are draining
• any bleeding
• tiredness or weakness
• shortness of breath -
Liver problems.Increases in liver function test results are common with PURIXAN, but you can also develop severe liver problems with PURIXAN that can lead to death.
Your healthcare provider may tell you to stop taking PURIXAN if you develop liver problems. Tell your healthcare provider right away if you develop any of the following symptoms of a liver problem during treatment with PURIXAN:
• decreased appetite
• diarrhea
• nausea or vomiting
• yellowing of your skin or the whites of your eyes
• a build-up of fluid in your stomach-area (ascites)
Less common side effects of PURIXAN include:loss of appetite, nausea, vomiting, diarrhea, generally do not feel well and rash.
Low blood sugar (hypoglycemia) can happen, especially in children under six years of age.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of PURIXAN.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store PURIXAN? - PURIXAN comes in a bottle with a child-resistant cap.
- Store PURIXAN between 59ºF to 77ºF (15ºC to 25ºC), in a dry place. Do not store above 25°C.
- Store the oral dispensing syringe in a clean place, with the medicine.
- PURIXAN oral suspension should be used within 8 weeks after opening the bottle. Dispose of (throw away) any unused medicine after 8 weeks.
- Do not use after the expiry date which is stated on the carton and the bottle after ‘EXP’.
- Keep the bottle tightly closed to prevent spoilage of the medicine and reduce the risk of accidental spillage.
- Keep PURIXAN out of the reach of children, preferably in a locked cupboard. If a child accidentally takes PURIXAN, it could cause death.
- This medicine should not be disposed of in wastewater or household waste. Ask your pharmacist how to dispose of (throw away) PURIXAN that is no longer needed.
General information about the safe and effective use of PURIXAN.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use PURIXAN for a condition for which it was not prescribed. Do not give PURIXAN to other people, even if they have the same symptoms you have. It could harm them. You can ask your healthcare provider or pharmacist for information about PURIXAN that is written for health professionals.What are the ingredients in PURIXAN?
Active ingredient: mercaptopurine
Inactive ingredients: xanthan gum, aspartame, concentrated raspberry juice, sucrose, ethyl parahydroxybenzoate sodium, methyl parahydroxybenzoate sodium, potassium sorbate, sodium hydroxide and purified water.
Manufactured by: Nova Laboratories Ltd, Leicester, LE18 4YL, United Kingdom
Manufactured for: Rare Disease Therapeutics, Inc., 2550 Meridian Blvd. Suite 150, Franklin, TN 37067
For more information, go to www.purixan-us.com.
Part Number: D001354/1This Patient information has been approved by the U.S. Food and Drug Administration
Revised: April 2020
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
PURIXAN® (pure-ee-zan)
mercaptopurine)
oral suspension
20 mg/mLRead these Instructions for Use before you start taking PURIXAN, and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Important information about measuring PURIXAN oral suspension
• Always use the oral dispensing syringe provided with your PURIXAN oral suspension to make sure you measure the right amount.
• You will be provided:- 1 bottle of PURIXAN oral suspension
- 1 bottle adapter
- 2 oral dispensing syringes (one 1 mL and one 5 mL)
If you did not receive an oral dispensing syringe with your PURIXAN oral suspension, ask your pharmacist to give you one.
Important Information You Need to Know Before Administering PURIXAN:
You will need disposable gloves.
1. Wash your hands well with soap and water before and after administering a dose. 2. Put on disposable gloves before handling PURIXAN. 3. Shake the bottle vigorously for at least 30 seconds to make sure that the medicine is well mixed (See Figure A).  Figure A
Figure A4. Remove the child-resistant bottle cap (See Figure B). 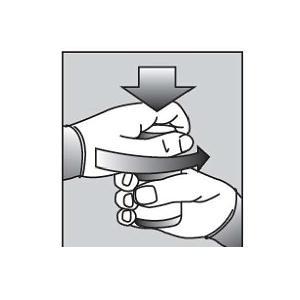 Figure B
Figure B5. Push the ribbed end of the bottle adapter into the neck of the bottle until it is firmly in place. The bottom edge of the adapter should fully contact the top rim of the bottle (See Figure C). Do not remove the adapter from the bottle after it is inserted.
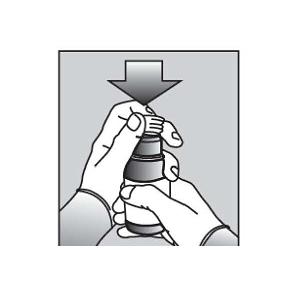 Figure C
Figure CPreparing a dose of PURIXAN: 6. Hold the bottle upright. Remove the bottle cap by turning in the direction of the arrow (See Figure B).
7. Push the tip of the oral dispensing syringe into the hole in the bottle adapter (See Figure D and Figure E). 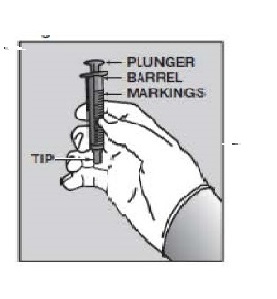 Figure D
Figure D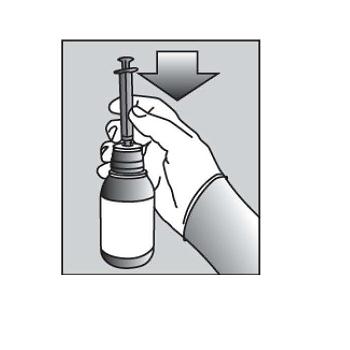 Figure E
Figure E8. Turn the bottle upside down (See Figure F). 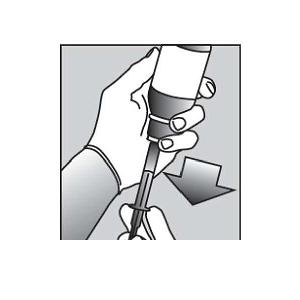 Figure F
Figure F9. Pull back slowly on the plunger of the oral dispensing syringe to withdraw the prescribed dose of PURIXAN. Pull the plunger back to the mL mark of the syringe that corresponds to the dose prescribed (Figure F). If you are not sure about how much medicine to draw into the oral dispensing syringe, always ask your doctor, pharmacist or nurse for advice.
10. Leave the oral dispensing syringe in the bottle adapter and turn the bottle right-side up. Place the bottle onto a flat surface. Hold the oral dispensing syringe by the barrel and carefully remove it from the adapter. Do not hold the oral dispensing syringe by the plunger, because the plunger may come out. 11. Place the tip of the oral dispensing syringe in your mouth and aim the tip toward the inside of your cheek. 12. Gently squirt the PURIXAN oral suspension into your mouth by pushing on the plunger until the oral dispensing syringe is empty. Swallow the medicine. - Do notforcefully push on the plunger.
- Do notsquirt the medicine to the back of your mouth or throat. This may cause you to choke.
13. Remove the oral dispensing syringe from your mouth. 14. Swallow the dose of oral suspension then drink some water, making sure no medicine is left in your mouth. 15. Put the cap back on the bottle with the adapter left in place. Close the cap tightly. 16. Wash the oral dispensing syringe with warm soapy water and rinse well. Hold the oral dispensing syringe under water and move the plunger up and down several times to make sure the inside of the oral dispensing syringe is clean. Let the oral dispensing syringe dry completely before you use it again for dosing. Do not throw away the oral dispensing syringe after use. Ingredients in PURIXAN
Active ingredient: mercaptopurine
Inactive ingredients: xanthan gum, aspartame, concentrated raspberry juice, sucrose, ethyl parahydroxybenzoate sodium, methyl parahydroxybenzoate sodium, potassium sorbate, sodium hydroxide and purified water.
Storing PURIXAN
- Store PURIXAN between 59ºF to 77ºF (15ºC to 25ºC), in a dry place. Do not store above 25°C.
- Store the oral dispensing syringe in a clean place, with the medicine.
- PURIXAN oral suspension should be used within 8 weeks after opening the bottle. Dispose of (throw away) any unused medicine after 8 weeks.
- Do not use after the expiry date which is stated on the carton and the bottle after ‘EXP’.
- Keep the bottle tightly closed to prevent spoilage of the medicine and reduce the risk of accidental spillage.
- Keep PURIXAN oral suspension and all medicines out of the reach of children, preferably in a locked cupboard. If a child accidentally takes PURIXAN, it could cause death.Ask your pharmacist how to dispose of (throw away) PURIXAN that is no longer needed.
- Ask your pharmacist how to dispose of PURIXAN that is expired or no longer required. Medicines should not be disposed of via wastewater or household waste.
Clean Up Spillage of PURIXAN
Use appropriate personal protective equipment (disposable gloves and eye protection). Mop up and contain spill material in a compatible container. Wash your hands thoroughly afterwards.PURIXAN contact with Skin, Eyes, or Clothes
- Remove and launder contaminated clothing.
- Wash skin or eyes immediately with water.
Contact with skin or eyes can cause hypersensitive reactions resulting in rash, redness, itching and inflammation. If symptoms appear, seek medical attention.
Manufactured by: Nova Laboratories Ltd, Leicester, LE18 4YL, United Kingdom
Manufactured for: Rare Disease Therapeutics, Inc., 2550 Meridian Blvd. Suite 150, Franklin, TN 37067
Part Number: D001112/1
This “Instructions for Use” has been approved by the U.S. Food and Drug Administration Revised: April 2020 - PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
PURIXAN
purixan suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62484-0020 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Mercaptopurine (UNII: E7WED276I5) (MERCAPTOPURINE ANHYDROUS - UNII:PKK6MUZ20G) Mercaptopurine 20 mg in 1 mL Product Characteristics Color Score Shape Size Flavor Raspberry Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62484-0020-2 1 in 1 CARTON 04/28/2014 1 NDC:62484-0020-1 100 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205919 04/28/2014 Labeler - Nova Laboratories, Ltd (230804692) Registrant - Rare Disease Therapeutics, Inc. (966133100) Establishment Name Address ID/FEI Business Operations Nova Laboratories, Ltd 230804692 manufacture(62484-0020)