Label: AMPICILLIN AND SULBACTAM- ampicillin sodium and sulbactam sodium injection, powder, for solution
- NDC Code(s): 71288-033-91
- Packager: Meitheal Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONnovaplus+TMPHARMACY BULK PACKAGE -Rx only - NOT FOR DIRECT INFUSION - To reduce the development of drug-resistant bacteria and maintain the effectiveness of Ampicillin and Sulbactam for ...
-
DESCRIPTION
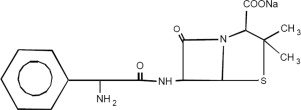
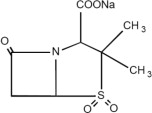
Ampicillin and Sulbactam for Injection, USP is an injectable antibacterial combination consisting of the semisynthetic antibacterial ampicillin sodium and the beta-lactamase inhibitor sulbactam ...
-
CLINICAL PHARMACOLOGY
General - Immediately after completion of a 15-minute intravenous infusion of ampicillin and sulbactam for injection, peak serum concentrations of ampicillin and sulbactam are attained ...
-
MICROBIOLOGY
Ampicillin is similar to benzyl penicillin in its bactericidal action against susceptible organisms during the stage of active multiplication. It acts through the inhibition of cell wall ...
-
INDICATIONS AND USAGE
Ampicillin and sulbactam for injection is indicated for the treatment of infections due to susceptible strains of the designated microorganisms in the conditions listed below. Skin and Skin ...
-
CONTRAINDICATIONS
The use of ampicillin and sulbactam for injection is contraindicated in individuals with a history of serious hypersensitivity reactions (e.g., anaphylaxis or Stevens-Johnson syndrome) to ...
-
WARNINGS
Hypersensitivity - Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients on penicillin therapy. These reactions are more apt to occur in ...
-
PRECAUTIONS
General - A high percentage of patients with mononucleosis who receive ampicillin develop a skin rash. Thus, ampicillin class antibacterial should not be administered to patients with ...
-
ADVERSE REACTIONS
Adult Patients - Ampicillin and sulbactam for injection is generally well tolerated. The following adverse reactions have been reported in clinical trials. Local Adverse Reactions - Pain at IM ...
-
OVERDOSAGE
Neurological adverse reactions, including convulsions, may occur with the attainment of high CSF levels of beta-lactams. Ampicillin may be removed from circulation by hemodialysis. The molecular ...
-
CLINICAL STUDIES
Skin and Skin Structure Infections in Pediatric Patients - Data from a controlled clinical trial conducted in pediatric patients provided evidence supporting the safety and efficacy of ...
-
DOSAGE AND ADMINISTRATION
Ampicillin and sulbactam for injection may be used for parenteral administration (following dilution). THE INTENT OF THIS PHARMACY BULK PACKAGE IS FOR PREPARATION OF SOLUTIONS FOR IV INFUSION ...
-
COMPATIBILITY, RECONSTITUTION AND STABILITY
Ampicillin and sulbactam for injection sterile powder is to be stored at or below 30°C (86°F) prior to reconstitution. When concomitant therapy with aminoglycosides is indicated, ampicillin and ...
-
DIRECTIONS FOR USE
Intravenous Administration - Directions for Proper Use of Pharmacy Bulk Package - Ampicillin and sulbactam for injection sterile powder for intravenous use may be reconstituted with any of the ...
-
HOW SUPPLIED
Ampicillin and Sulbactam for Injection, USP (ampicillin sodium/sulbactam sodium) Pharmacy Bulk Package is supplied as follows: NDCAmpicillin and Sulbactam for Injection, USPPackage ...
-
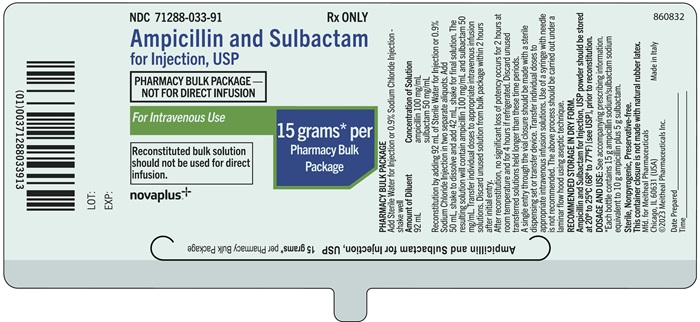
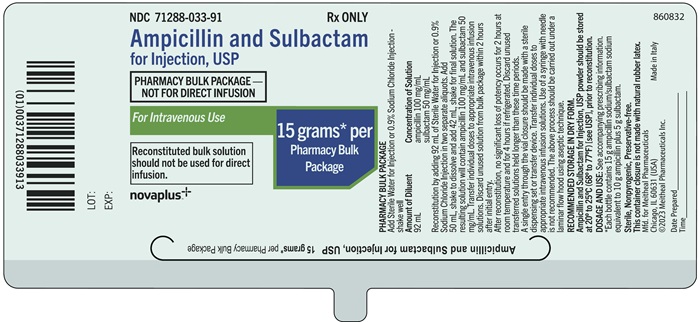
Principal Display Panel – Ampicillin and Sulbactam for Injection, USP 15 gram Bottle LabelNDC 71288-033-91 - Rx ONLY - Ampicillin and Sulbactam for Injection, USP - PHARMACY BULK PACKAGE - NOT FOR DIRECT INFUSION - For Intravenous Use - 15 grams* per Pharmacy Bulk Package - Reconstituted ...
-
Principal Display Panel – Ampicillin and Sulbactam for Injection, USP 15 gram Bottle CartonNDC 71288-033-91 - Ampicillin and Sulbactam for Injection, USP - PHARMACY BULK PACKAGE - NOT FOR DIRECT INFUSION - For Intravenous Use - 15 grams* per Pharmacy Bulk Package - Rx ONLY - Reconstituted ...
-
INGREDIENTS AND APPEARANCEProduct Information