Label: TOLTERODINE TARTRATE tablet, film coated
- NDC Code(s): 59762-0170-1, 59762-0170-6, 59762-0800-2, 59762-0800-6
- Packager: Greenstone LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated September 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
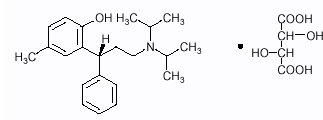
Tolterodine tartrate tablets contain tolterodine tartrate. The active moiety, tolterodine, is a muscarinic receptor antagonist. The chemical name of tolterodine tartrate is ...
-
CLINICAL PHARMACOLOGY Tolterodine is a competitive muscarinic receptor antagonist. Both urinary bladder contraction and salivation are mediated via cholinergic muscarinic receptors. After oral administration ...
-
CLINICAL STUDIES Tolterodine tartrate tablets were evaluated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency in four randomized, double-blind ...
-
INDICATIONS AND USAGE Tolterodine tartrate tablets are indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency.
-
CONTRAINDICATIONS Tolterodine tartrate tablets are contraindicated in patients with urinary retention, gastric retention, or uncontrolled narrow-angle glaucoma. Tolterodine tartrate tablets are also contraindicated ...
-
WARNINGS Anaphylaxis and angioedema requiring hospitalization and emergency medical treatment have occurred with the first or subsequent doses of tolterodine tartrate tablets. In the event of difficulty in ...
-
PRECAUTIONS General - Risk of Urinary Retention and Gastric Retention - Tolterodine tartrate tablets should be administered with caution to patients with clinically significant bladder outflow ...
-
ADVERSE REACTIONS The Phase 2 and 3 clinical trial program for tolterodine tartrate tablets included 3071 patients who were treated with tolterodine tartrate tablets (N=2133) or placebo (N=938). The patients were ...
-
OVERDOSAGE A 27-month-old child who ingested 5 to 7 tolterodine tartrate tablets 2 mg was treated with a suspension of activated charcoal and was hospitalized overnight with symptoms of dry mouth. The child ...
-
DOSAGE AND ADMINISTRATION The initial recommended dose of tolterodine tartrate tablets is 2 mg twice daily. The dose may be lowered to 1 mg twice daily based on individual response and tolerability. For patients with ...
-
HOW SUPPLIED Tolterodine Tartrate Tablets 1 mg (white, round, biconvex, film-coated tablets engraved with arcs above and below the letters “TO”) and Tolterodine Tartrate Tablets 2 mg (white, round, biconvex ...
-
Patient Information TOLTERODINE TARTRATE TABLETS - (tolterodine tartrate tablets) Read the Patient Information that comes with tolterodine tartrate tablets before you start using it and each time you get a refill ...
-
PRINCIPAL DISPLAY PANEL – 1 mg NDC 59762-0170-6 - 60 Tablets - GREENSTONE® BRAND - tolterodine - tartrate tablets - 1 mg - Rx only - Store at 25°C (77°F); excursions - permitted to 15-30°C (59-86°F) [see USP Controlled Room ...
-
PRINCIPAL DISPLAY PANEL – 2 mg NDC 59762-0800-2 - 60 Tablets - GREENSTONE® BRAND - tolterodine - tartrate tablets - 2 mg - Rx only - Store at 25°C (77°F); excursions - permitted to 15-30°C (59-86°F) [see USP Controlled Room ...
-
INGREDIENTS AND APPEARANCEProduct Information