Label: METHYLDOPA tablet
- NDC Code(s): 64980-571-01, 64980-572-01
- Packager: Rising Pharma Holdings, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
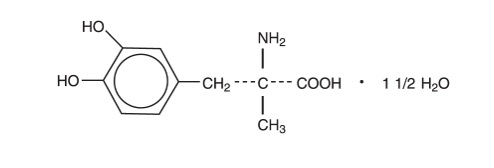
DESCRIPTIONMethyldopa is an antihypertensive and is the L-isomer of alpha-methyldopa. It is levo-3-(3,4-dihydroxyphenyl)-2-methylalanine sesquihydrate. Methyldopa is supplied as tablets for oral ...
-
CLINICAL PHARMACOLOGYMethyldopa is an aromatic-aminoacid decarboxylase inhibitor in animals and in man. Although the mechanism of action has yet to be conclusively demonstrated, the antihypertensive effect of ...
-
INDICATIONS & USAGEHypertension.
-
CONTRAINDICATIONSMethyldopa is contraindicated in patients: – with active hepatic disease, such as acute hepatitis and active cirrhosis. – with liver disorders previously associated with methyldopa therapy (see ...
-
WARNINGSIt is important to recognize that a positive Coombs test, hemolytic anemia, and liver disorders may occur with methyldopa therapy. The rare occurrences of hemolytic anemia or liver disorders ...
-
PRECAUTIONSGeneral - Methyldopa should be used with caution in patients with a history of previous liver disease or dysfunction (see WARNINGS). Some patients taking methyldopa experience clinical edema ...
-
ADVERSE REACTIONSSedation, usually transient, may occur during the initial period of therapy or whenever the dose is increased. Headache, asthenia, or weakness may be noted as early and transient symptoms ...
-
OVERDOSAGEAcute overdosage may produce acute hypotension with other responses attributable to brain and gastrointestinal malfunction (excessive sedation, weakness, bradycardia, dizziness, light-headedness ...
-
DOSAGE & ADMINISTRATIONAdults: Initiation of Therapy: The usual starting dosage of methyldopa tablets is 250 mg two to three times a day in the first 48 hours. The daily dosage then may be increased or decreased ...
-
HOW SUPPLIEDMethyldopa Tablets, USP are supplied as film-coated tablets containing either 250 mg or 500 mg of Methyldopa, USP. The 250 mg tablet is a beige, film-coated, round, biconvex, beveled-edge ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 64980-572-01 - Methyldopa Tablets, USP - 500 mg - 100 Tablets Rx Only
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 64980-571-01 - Methyldopa Tablets, USP - 250 mg - 100 Tablets Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information