Label: ROPIVACAINE HYDROCHLORIDE injection, solution
-
NDC Code(s):
67457-798-10,
67457-798-98,
67457-798-99,
67457-799-20, view more67457-799-98, 67457-799-99
- Packager: Mylan Institutional LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ROPIVACAINE HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for ROPIVACAINE HYDROCHLORIDE ...These highlights do not include all the information needed to use ROPIVACAINE HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for ROPIVACAINE HYDROCHLORIDE INJECTION.
ROPIVACAINE HYDROCHLORIDE injection, for epidural, perineural, or infiltration use
Initial U.S. Approval: 1996INDICATIONS AND USAGE
Ropivacaine hydrochloride injection is an amide local anesthetic indicated in adults for the production of local or regional anesthesia for surgery and for acute pain management. (1)
Surgical Anesthesia: epidural block for surgery including cesarean section; major nerve block; local infiltration (1)
Acute Pain Management: epidural continuous infusion or intermittent bolus, e.g., postoperative or labor; local infiltration (1)
DOSAGE AND ADMINISTRATION
- •
- See Table 1 for Dosage Recommendations (2.2)
DOSAGE FORMS AND STRENGTHS
- •
- Injection: 2 mg/mL (0.2%) in single-dose, ready-to-use, polypropylene flexible bag (3)
CONTRAINDICATIONS
History of hypersensitivity to local anesthetics of the amide type. (4)
WARNINGS AND PRECAUTIONS
- •
- Delay in proper management of dose-related toxicity, underventilation, and/or altered sensitivity may lead to the development of acidosis, cardiac arrest and, possibly, death. (5.1)
- •
- In performing ropivacaine hydrochloride blocks, unintended intravenous injection is possible and may result in cardiac arrhythmia or cardia arrest. (5.2)
- •
- Intra-articular infusions of local anesthetics may cause chondrolysis. Ropivacaine hydrochloride is not approved for this use. (5.3).
- •
- Signs of methemoglobinemia may occur. (5.4)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 5%) are hypotension, nausea, vomiting, bradycardia, fever, pain, postoperative complications, anemia, paresthesia, headache, pruritus, and back pain. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Agents structurally related to amide-type local anesthetics: Concurrent use may cause additive effects. (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2023
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosage Recommendations
2.3 Other Administration Considerations
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 General Warnings and Precautions
5.2 Unintended Intravenous Injection
5.3 Intra-Articular Infusions and Risk of Chondrolysis
5.4 Risk of Methemoglobinemia
5.5 Central Nervous System Toxicity
5.6 Epidural Anesthesia
5.7 Use in Brachial Plexus Block
5.8 Use in Peripheral Nerve Block
5.9 Use in Head and Neck Area
5.10 Use in Ophthalmic Surgery
5.11 Hepatic Disease
5.12 Cardiovascular Impairment
5.13 Risk of Additive Effects
5.14 Malignant Hyperthermia
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
10.1 Treatment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Epidural Administration in Surgery
14.2 Epidural Administration in Cesarean Section
14.3 Epidural Administration in Labor and Delivery
14.4 Epidural Administration in Postoperative Pain Management
14.5 Peripheral Nerve Block
14.6 Local Infiltration
16 How Supplied/Storage and Handling
17 PATIENT COUNSELING INFORMATION
17.1 Information for Patients and Caregivers
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE Ropivacaine hydrochloride injection is indicated for the production of local or regional anesthesia for surgery and for acute pain management. Surgical Anesthesia: epidural block for surgery ...
Ropivacaine hydrochloride injection is indicated for the production of local or regional anesthesia for surgery and for acute pain management.
Surgical Anesthesia: epidural block for surgery including cesarean section; major nerve block; local infiltration
Acute Pain Management: epidural continuous infusion or intermittent bolus, e.g., postoperative or labor; local infiltration
Close -
2 DOSAGE AND ADMINISTRATION 2.1 Important Administration Instructions There have been adverse event reports of chondrolysis in patients receiving intra-articular infusions of local anesthetics following arthroscopic and ...
2.1 Important Administration Instructions
There have been adverse event reports of chondrolysis in patients receiving intra-articular infusions of local anesthetics following arthroscopic and other surgical procedures. Ropivacaine hydrochloride injection is not approved for this use [see Warnings and Precautions (5.3)].
The rapid injection of a large volume of local anesthetic solution should be avoided and fractional (incremental) doses should always be used. The smallest dose and concentration required to produce the desired result should be administered.
The dose of any local anesthetic administered varies with the anesthetic procedure, the area to be anesthetized, the vascularity of the tissues, the number of neuronal segments to be blocked, the depth of anesthesia and degree of muscle relaxation required, the duration of anesthesia desired, individual tolerance, and the physical condition of the patient. Patients in poor general condition due to aging or other compromising factors such as partial or complete heart conduction block, advanced liver disease or severe renal dysfunction require special attention although regional anesthesia is frequently indicated in these patients. To reduce the risk of potentially serious adverse reactions, attempts should be made to optimize the patient's condition before major blocks are performed, and the dosage should be adjusted accordingly.
Use an adequate test dose (3 mL to 5 mL of a short acting local anesthetic solution containing epinephrine) prior to induction of complete block. This test dose should be repeated if the patient is moved in such a fashion as to have displaced the epidural catheter. Allow adequate time for onset of anesthesia following administration of each test dose.
These products are intended for single dose and are free from preservatives. Any solution remaining from an opened container should be discarded promptly. In addition, continuous infusion bottles should not be left in place for more than 24 hours.
2.2 Dosage Recommendations
Table 1 Dosage Recommendations
Conc.
Volume
Dose
Onset
Duration
mg/mL
(%)
mL
mg
min
hours
SURGICAL ANESTHESIA
Lumbar Epidural
Administration
Surgery
5
(0.5%)
15 to 30
75 to 150
15 to 30
2 to 4
7.5
(0.75%)
15 to 25
113 to 188
10 to 20
3 to 5
10
(1%)
15 to 20
150 to 200
10 to 20
4 to 6
Lumbar Epidural
Administration
Cesarean Section
5
(0.5%)
20 to 30
100 to 150
15 to 25
2 to 4
7.5
(0.75%)
15 to 20
113 to 150
10 to 20
3 to 5
Thoracic Epidural
Administration
Surgery
5
(0.5%)
5 to 15
25 to 75
10 to 20
n/a*
7.5
(0.75%)
5 to 15
38 to 113
10 to 20
n/a*
Major Nerve Block†
(e.g., brachial plexus block)
5
(0.5%)
35 to 50
175 to 250
15 to 30
5 to 8
7.5
(0.75%)
10 to 40
75 to 300
10 to 25
6 to 10
Field Block
(e.g., minor nerve blocks and infiltration)
5
(0.5%)
1 to 40
5 to 200
1 to 15
2 to 6
LABOR PAIN MANAGEMENT
Lumbar Epidural Administration
Initial Dose
2
(0.2%)
10 to 20
20 to 40
10 to 15
0.5 to 1.5
Continuous infusion‡
2
- (0.2%)
6 to 14 mL/h
12 to 28 mg/h
n/a*
n/a*
Incremental injections (top-up) ‡
2
(0.2%)
10 to 15 mL/h
20 to 30 mg/h
n/a*
n/a*
POSTOPERATIVE PAIN MANAGEMENT
Lumbar Epidural Administration Continuous infusion§
2
(0.2%)
6 to 14 mL/h
12 to 28 mg/h
n/a*
n/a*
Thoracic Epidural Administration
Continuous infusion§
2
(0.2%)
6 to 14 mL/h
12 to 28 mg/h
n/a*
n/a*
Infiltration
(e.g., minor nerve block)
2
(0.2%)
1 to 100
2 to 200
1 to 5
2 to 6
5
(0.5%)
1 to 40
5 to 200
1 to 5
2 to 6
* = Not Applicable
- † = The dose for a major nerve block must be adjusted according to site of administration and patient status. Supraclavicular brachial plexus blocks may be associated with a higher frequency of serious adverse reactions, regardless of the local anesthetic used [see Warnings and Precautions (5.7)].
- ‡ = Median dose of 21 mg per hour was administered by continuous infusion or by incremental injections (top-ups) over a median delivery time of 5.5 hours.
- § = Cumulative doses up to 770 mg of ropivacaine hydrochloride injection over 24 hours (intraoperative block plus postoperative infusion); Continuous epidural infusion at rates up to 28 mg per hour for 72 hours have been well tolerated in adults, i.e., 2016 mg plus surgical dose of approximately 100 mg to 150 mg as top-up.
The doses in the table are those considered to be necessary to produce a successful block and should be regarded as guidelines for use in adults. Individual variations in onset and duration occur. The figures reflect the expected average dose range needed. For other local anesthetic techniques standard current textbooks should be consulted.
When prolonged blocks are used, either through continuous infusion or through repeated bolus administration, the risks of reaching a toxic plasma concentration or inducing local neural injury must be considered. Experience to date indicates that a cumulative dose of up to 770 mg ropivacaine hydrochloride injection administered over 24 hours is well tolerated in adults when used for postoperative pain management: i.e., 2016 mg. Caution should be exercised when administering ropivacaine hydrochloride injection for prolonged periods of time, e.g., >70 hours in debilitated patients.
For treatment of postoperative pain, the following technique can be recommended: If regional anesthesia was not used intraoperatively, then an initial epidural block with 5 to 7 mL ropivacaine hydrochloride injection is induced via an epidural catheter. Analgesia is maintained with an infusion of ropivacaine hydrochloride injection, 2 mg/mL (0.2%). Clinical studies have demonstrated that infusion rates of 6 mL to 14 mL (12 mg to 28 mg) per hour provide adequate analgesia with nonprogressive motor block. With this technique a significant reduction in the need for opioids was demonstrated. Clinical experience supports the use of ropivacaine hydrochloride injection epidural infusions for up to 72 hours.
Close2.3 Other Administration Considerations
Disinfecting agents containing heavy metals, which cause release of respective ions (mercury, zinc, copper, etc.) should not be used for skin or mucous membrane disinfection since they have been related to incidents of swelling and edema.
The solubility of ropivacaine is limited at pH above 6. Thus, care must be taken as precipitation may occur if ropivacaine hydrochloride injection is mixed with alkaline solutions. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Solutions which are discolored or which contain particulate matter should not be administered.
-
3 DOSAGE FORMS AND STRENGTHS Ropivacaine hydrochloride injection, USP is a clear, colorless, preservative-free solution available as: Ropivacaine Hydrochloride Injection, USP Single-Dose, Ready-to-Use, polypropylene flexible ...
Ropivacaine hydrochloride injection, USP is a clear, colorless, preservative-free solution available as:
Ropivacaine Hydrochloride Injection, USP Single-Dose, Ready-to-Use, polypropylene flexible bag
-
-
- •
- 0.2%, 200 mg per 100 mL (2 mg/mL), 100 mL single-dose, ready-to-use, polypropylene flexible bag
- •
- 0.2%, 400 mg per 200 mL (2 mg/mL), 200 mL single-dose, ready-to-use, polypropylene flexible bag
-
4 CONTRAINDICATIONS Ropivacaine hydrochloride injection is contraindicated in patients with a known hypersensitivity to ropivacaine or to any local anesthetic agent of the amide type.
Ropivacaine hydrochloride injection is contraindicated in patients with a known hypersensitivity to ropivacaine or to any local anesthetic agent of the amide type.
Close -
5 WARNINGS AND PRECAUTIONS 5.1 General Warnings and Precautions - Prior to receiving major blocks the general condition of the patient should be optimized and the patient should have an IV line inserted. All necessary ...
5.1 General Warnings and Precautions
Prior to receiving major blocks the general condition of the patient should be optimized and the patient should have an IV line inserted. All necessary precautions should be taken to avoid intravascular injection. Local anesthetics should only be administered by clinicians who are well versed in the diagnosis and management of dose-related toxicity and other acute emergencies which might arise from the block to be employed, and then only after insuring the immediate (without delay) availability of oxygen, other resuscitative drugs, cardiopulmonary resuscitative equipment, and the personnel resources needed for proper management of toxic reactions and related emergencies [see Adverse Reactions (6) and Overdosage (10.1)]. Delay in proper management of dose-related toxicity, underventilation from any cause, and/or altered sensitivity may lead to the development of acidosis, cardiac arrest and, possibly, death.
The safe and effective use of local anesthetics depends on proper dosage, correct technique, adequate precautions and readiness for emergencies.
Resuscitative equipment, oxygen and other resuscitative drugs should be available for immediate use [see Adverse Reactions (6)]. The lowest dosage that results in effective anesthesia should be used to avoid high plasma levels and serious adverse events. Injections should be made slowly and incrementally, with frequent aspirations before and during the injection to avoid intravascular injection. When a continuous catheter technique is used, syringe aspirations should also be performed before and during each supplemental injection. During the administration of epidural anesthesia, it is recommended that a test dose of a local anesthetic with a fast onset be administered initially and that the patient be monitored for central nervous system and cardiovascular toxicity, as well as for signs of unintended intrathecal administration before proceeding. When clinical conditions permit, consideration should be given to employing local anesthetic solutions, which contain epinephrine for the test dose because circulatory changes compatible with epinephrine may also serve as a warning sign of unintended intravascular injection. An intravascular injection is still possible even if aspirations for blood are negative. Administration of higher than recommended doses of ropivacaine hydrochloride to achieve greater motor blockade or increased duration of sensory blockade may result in cardiovascular depression, particularly in the event of inadvertent intravascular injection. Tolerance to elevated blood levels varies with the physical condition of the patient. Debilitated, elderly patients and acutely ill patients should be given reduced doses commensurate with their age and physical condition. Local anesthetics should also be used with caution in patients with hypotension, hypovolemia or heart block.
Solutions of ropivacaine hydrochloride should not be used for the production of obstetrical paracervical block anesthesia, retrobulbar block, or spinal anesthesia (subarachnoid block) due to insufficient data to support such use. Intravenous regional anesthesia (bier block) should not be performed due to a lack of clinical experience and the risk of attaining toxic blood levels of ropivacaine.
It is essential that aspiration for blood, or cerebrospinal fluid (where applicable), be done prior to injecting any local anesthetic, both the original dose and all subsequent doses, to avoid intravascular or subarachnoid injection. However, a negative aspiration does not ensure against an intravascular or subarachnoid injection.
5.2 Unintended Intravenous Injection
In performing ropivacaine hydrochloride blocks, unintended intravenous injection is possible and may result in cardiac arrhythmia or cardiac arrest. The potential for successful resuscitation has not been studied in humans. There have been rare reports of cardiac arrest during the use of ropivacaine hydrochloride for epidural anesthesia or peripheral nerve blockade, the majority of which occurred after unintentional accidental intravascular administration in elderly patients and in patients with concomitant heart disease. In some instances, resuscitation has been difficult. Should cardiac arrest occur, prolonged resuscitative efforts may be required to improve the probability of a successful outcome.
Ropivacaine hydrochloride should be administered in incremental doses. It is not recommended for emergency situations, where a fast onset of surgical anesthesia is necessary. Historically, pregnant patients were reported to have a high risk for cardiac arrhythmias, cardiac/circulatory arrest and death when 0.75% bupivacaine (another member of the amino amide class of local anesthetics) was inadvertently rapidly injected intravenously.
5.3 Intra-Articular Infusions and Risk of Chondrolysis
Intra-articular infusions of local anesthetics following arthroscopic and other surgical procedures is an unapproved use, and there have been post-marketing reports of chondrolysis in patients receiving such infusions. The majority of reported cases of chondrolysis have involved the shoulder joint; cases of gleno-humeral chondrolysis have been described in pediatric and adult patients following intra-articular infusions of local anesthetics with and without epinephrine for periods of 48 to 72 hours. There is insufficient information to determine whether shorter infusion periods are not associated with these findings. The time of onset of symptoms, such as joint pain, stiffness and loss of motion can be variable, but may begin as early as the 2nd month after surgery. Currently, there is no effective treatment for chondrolysis; patients who experienced chondrolysis have required additional diagnostic and therapeutic procedures and some required arthroplasty or shoulder replacement.
5.4 Risk of Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure, and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue ropivacaine hydrochloride and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
5.5 Central Nervous System Toxicity
Careful and constant monitoring of cardiovascular and respiratory vital signs (adequacy of ventilation) and the patient's state of consciousness should be performed after each local anesthetic injection. It should be kept in mind at such times that restlessness, anxiety, incoherent speech, light-headedness, numbness and tingling of the mouth and lips, metallic taste, tinnitus, dizziness, blurred vision, tremors, twitching, depression, or drowsiness may be early warning signs of central nervous system toxicity.
5.6 Epidural Anesthesia
A well-known risk of epidural anesthesia may be an unintentional subarachnoid injection of local anesthetic. Two clinical studies have been performed to verify the safety of ropivacaine hydrochloride at a volume of 3 mL injected into the subarachnoid space since this dose represents an incremental epidural volume that could be unintentionally injected. The 15 and 22.5 mg doses injected resulted in sensory levels as high as T5 and T4, respectively. Anesthesia to pinprick started in the sacral dermatomes in 2 to 3 minutes, extended to the T10 level in 10 to 13 minutes and lasted for approximately 2 hours. The results of these two clinical studies showed that a 3 mL dose did not produce any serious adverse events when spinal anesthesia blockade was achieved.
During epidural administration, ropivacaine hydrochloride should be administered in incremental doses of 3 to 5 mL with sufficient time between doses to detect toxic manifestations of unintentional intravascular or intrathecal injection. Syringe aspirations should also be performed before and during each supplemental injection in continuous (intermittent) catheter techniques. An intravascular injection is still possible even if aspirations for blood are negative. During the administration of epidural anesthesia, it is recommended that a test dose be administered initially and the effects monitored before the full dose is given. When clinical conditions permit, the test dose should contain an appropriate dose of epinephrine to serve as a warning of unintentional intravascular injection. If injected into a blood vessel, this amount of epinephrine is likely to produce a transient "epinephrine response" within 45 seconds, consisting of an increase in heart rate and systolic blood pressure, circumoral pallor, palpitations and nervousness in the unsedated patient. The sedated patient may exhibit only a pulse rate increase of 20 or more beats per minute for 15 or more seconds. Therefore, following the test dose, the heart should be continuously monitored for a heart rate increase. Patients on beta-blockers may not manifest changes in heart rate, but blood pressure monitoring can detect a rise in systolic blood pressure. A test dose of a short-acting amide anesthetic such as lidocaine is recommended to detect an unintentional intrathecal administration. This will be manifested within a few minutes by signs of spinal block (e.g., decreased sensation of the buttocks, paresis of the legs, or, in the sedated patient, absent knee jerk). An intravascular or subarachnoid injection is still possible even if results of the test dose are negative. The test dose itself may produce a systemic toxic reaction, high spinal or epinephrine-induced cardiovascular effects.
5.7 Use in Brachial Plexus Block
Ropivacaine plasma concentrations may approach the threshold for central nervous system toxicity after the administration of 300 mg of ropivacaine for brachial plexus block. Caution should be exercised when using the 300 mg dose [see Overdosage (10)].
The dose for a major nerve block must be adjusted according to the site of administration and patient status. Supraclavicular brachial plexus blocks may be associated with a higher frequency of serious adverse reactions, regardless of the local anesthetic used.
5.8 Use in Peripheral Nerve Block
Major peripheral nerve blocks may result in the administration of a large volume of local anesthetic in highly vascularized areas, often close to large vessels where there is an increased risk of intravascular injection and/or rapid systemic absorption, which can lead to high plasma concentrations.
5.9 Use in Head and Neck Area
Small doses of local anesthetics injected into the head and neck area may produce adverse reactions similar to systemic toxicity seen with unintentional intravascular injections of larger doses. The injection procedures require the utmost care. Confusion, convulsions, respiratory depression, and/or respiratory arrest, and cardiovascular stimulation or depression have been reported. These reactions may be due to intra-arterial injection of the local anesthetic with retrograde flow to the cerebral circulation. Patients receiving these blocks should have their circulation and respiration monitored and be constantly observed. Resuscitative equipment and personnel for treating adverse reactions should be immediately available. Dosage recommendations should not be exceeded [see Dosage and Administration (2.2)].
5.10 Use in Ophthalmic Surgery
The use of ropivacaine hydrochloride in retrobulbar blocks for ophthalmic surgery has not been studied. Until appropriate experience is gained, the use of ropivacaine hydrochloride for such surgery is not recommended.
5.11 Hepatic Disease
Because amide-type local anesthetics such as ropivacaine are metabolized by the liver, these drugs, especially repeat doses, should be used cautiously in patients with hepatic disease. Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at a greater risk of developing toxic plasma concentrations.
5.12 Cardiovascular Impairment
Local anesthetics should also be used with caution in patients with impaired cardiovascular function because they may be less able to compensate for functional changes associated with the prolongation of A-V conduction produced by these drugs.
5.13 Risk of Additive Effects
Ropivacaine hydrochloride should be used with caution in patients receiving other local anesthetics or agents structurally related to amide-type local anesthetics, since the toxic effects of these drugs are additive. [See Drug Interactions 7]
Patients treated with class III antiarrhythmic drugs (e.g., amiodarone) should be under close surveillance and ECG monitoring considered, since cardiac effects may be additive. [See Drug Interactions 7]
Close5.14 Malignant Hyperthermia
Many drugs used during the conduct of anesthesia are considered potential triggering agents for malignant hyperthermia (MH). Amide-type local anesthetics are not known to trigger this reaction. However, since the need for supplemental general anesthesia cannot be predicted in advance, it is suggested that a standard protocol for MH management should be available.
-
6 ADVERSE REACTIONS Reactions to ropivacaine are characteristic of those associated with other amide-type local anesthetics. A major cause of adverse reactions to this group of drugs may be associated with excessive ...
Reactions to ropivacaine are characteristic of those associated with other amide-type local anesthetics. A major cause of adverse reactions to this group of drugs may be associated with excessive plasma levels, which may be due to overdosage, unintentional intravascular injection or slow metabolic degradation.
The reported adverse events are derived from clinical studies conducted in the U.S. and other countries. The reference drug was usually bupivacaine. The studies used a variety of premedications, sedatives, and surgical procedures of varying length. A total of 3,988 patients have been exposed to ropivacaine hydrochloride at concentrations up to 1% in clinical trials. Each patient was counted once for each type of adverse event.
Because clinical trials are conducted under widely conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Incidence ≥ 5%
For the indications of epidural administration in surgery, cesarean section, postoperative pain management, peripheral nerve block, and local infiltration, the following treatment-emergent adverse events were reported with an incidence of ≥ 5% in all clinical studies (N=3988): hypotension (37%), nausea (24.8%), vomiting (11.6%), bradycardia (9.3%), fever (9.2%), pain (8%), postoperative complications (7.1%), anemia (6.1%), paresthesia (5.6%), headache (5.1%), pruritus (5.1%), and back pain (5%).
Incidence 1 to 5%
Urinary retention, dizziness, rigors, hypertension, tachycardia, anxiety, oliguria, hypoesthesia, chest pain, hypokalemia, dyspnea, cramps, and urinary tract infection.
Incidence in Controlled Clinical Trials
The reported adverse events are derived from controlled clinical studies with ropivacaine hydrochloride (concentrations ranged from 0.125% to 1% for ropivacaine hydrochloride and 0.25% to 0.75% for bupivacaine) in the U.S. and other countries involving 3,094 patients. Table 2 and Table 3 list adverse events (number and percentage) that occurred in at least 1% of ropivacaine hydrochloride-treated patients in these studies. The majority of patients receiving concentrations higher than 5 mg/mL (0.5%) were treated with ropivacaine hydrochloride.
Table 2 Adverse Events Reported in ≥1% of Adult Patients Receiving Regional or Local Anesthesia (Surgery, Labor, Cesarean Section, Postoperative Pain Management, Peripheral Nerve Block and Local Infiltration)
Adverse Reaction
RopivacaineHydrochloride
total N=1661
Bupivacaine
total N=1433
N
(%)
N
(%)
Hypotension
536
(32.3)
408
(28.5)
Nausea
283
(17)
207
(14.4)
Vomiting
117
(7)
88
(6.1)
Bradycardia
96
(5.8)
73
(5.1)
Headache
84
(5.1)
68
(4.7)
Paresthesia
82
(4.9)
57
(4)
Back pain
73
(4.4)
75
(5.2)
Pain
71
(4.3)
71
(5)
Pruritus
63
(3.8)
40
(2.8)
Fever
61
(3.7)
37
(2.6)
Dizziness
42
(2.5)
23
(1.6)
Rigors (Chills)
42
(2.5)
24
(1.7)
Postoperative complications
41
(2.5)
44
(3.1)
Hypoesthesia
27
(1.6)
24
(1.7)
Urinary retention
23
(1.4)
20
(1.4)
Progression of labor poor/failed
23
(1.4)
22
(1.5)
Anxiety
21
(1.3)
11
(0.8)
Breast disorder, breast-feeding
21
(1.3)
12
(0.8)
Rhinitis
18
(1.1)
13
(0.9)
Table 3 Adverse Events Reported in ≥1% of Fetuses or Neonates of Mothers Who Received Regional Anesthesia (Cesarean Section and Labor Studies)
Adverse Reaction
Ropivacaine Hydrochloride
total N=639
Bupivacaine
total N=573
N
(%)
N
(%)
Fetal bradycardia
77
(12.1)
68
(11.9)
Neonatal jaundice
49
(7.7)
47
(8.2)
Neonatal complication-NOS
42
(6.6)
38
(6.6)
Apgar score low
18
(2.8)
14
(2.4)
Neonatal respiratory disorder
17
(2.7)
18
(3.1)
Neonatal tachypnea
14
(2.2)
15
(2.6)
Neonatal fever
13
(2)
14
(2.4)
Fetal tachycardia
13
(2)
12
(2.1)
Fetal distress
11
(1.7)
10
(1.7)
Neonatal infection
10
(1.6)
8
(1.4)
Neonatal hypoglycemia
8
(1.3)
16
(2.8)
Incidence <1%
The following adverse events were reported during the ropivacaine hydrochloride clinical program in more than one patient (N=3988), occurred at an overall incidence of <1%, and were considered relevant:
Application Site Reactions - injection site pain
Cardiovascular System - vasovagal reaction, syncope, postural hypotension, non-specific ECG abnormalities
Female Reproductive - poor progression of labor, uterine atony
Gastrointestinal System - fecal incontinence, tenesmus, neonatal vomiting
General and Other Disorders - hypothermia, malaise, asthenia, accident and/or injury
Hearing and Vestibular - tinnitus, hearing abnormalities
Heart Rate and Rhythm - extrasystoles, non-specific arrhythmias, atrial fibrillation
Liver and Biliary System - jaundice
Metabolic Disorders - hypomagnesemia
Musculoskeletal System - myalgia
Myo/Endo/Pericardium - ST segment changes, myocardial infarction
Nervous System - tremor, Horner’s syndrome, paresis, dyskinesia, neuropathy, vertigo, coma, convulsion, hypokinesia, hypotonia, ptosis, stupor
Psychiatric Disorders - agitation, confusion, somnolence, nervousness, amnesia, hallucination, emotional lability, insomnia, nightmares
Respiratory System - bronchospasm, coughing
Skin Disorders - rash, urticaria
Urinary System Disorders - urinary incontinence, micturition disorder
Vascular - deep vein thrombosis, phlebitis, pulmonary embolism
Vision - vision abnormalities
For the indication epidural anesthesia for surgery, the 15 most common adverse events were compared between different concentrations of ropivacaine hydrochloride and bupivacaine. Table 4 is based on data from trials in the U.S. and other countries where ropivacaine hydrochloride was administered as an epidural anesthetic for surgery.
Table 4 Common Events (Epidural Administration)
- Adverse Reaction
- Ropivacaine Hydrochloride
- Bupivacaine
- 5 mg/mL
- total N=256
- 7.5 mg/mL
- total N=297
- 10 mg/mL
- total N=207
- 5 mg/mL
- total N=236
- 7.5 mg/mL
- total N=174
- N
- (%)
- N
- (%)
- N
- (%)
- N
- (%)
- N
- (%)
- hypotension
- 99
- (38.7)
- 146
- (49.2)
- 113
- (54.6)
- 91
- (38.6)
- 89
- (51.1)
- nausea
- 34
- (13.3)
- 68
- (22.9)
- 41
- (17.4)
- 36
- (20.7)
- bradycardia
- 29
- (11.3)
- 58
- (19.5)
- 40
- (19.3)
- 32
- (13.6)
- 25
- (14.4)
- back pain
- 18
- (7)
- 23
- (7.7)
- 34
- (16.4)
- 21
- (8.9)
- 23
- (13.2)
- vomiting
- 18
- (7)
- 33
- (11.1)
- 23
- (11.1)
- 19
- (8.1)
- 14
- (8)
- headache
- 12
- (4.7)
- 20
- (6.7)
- 16
- (7.7)
- 13
- (5.5)
- 9
- (5.2)
- fever
- 8
- (3.1)
- 5
- (1.7)
- 18
- (8.7)
- 11
- (4.7)
- chills
- 6
- (2.3)
- 7
- (2.4)
- 6
- (2.9)
- 4
- (1.7)
- 3
- (1.7)
- urinary retention
- 5
- (2)
- 8
- (2.7)
- 10
- (4.8)
- 10
- (4.2)
- paresthesia
- 5
- (2)
- 10
- (3.4)
- 5
- (2.4)
- 7
- (3)
- pruritus
- 14
- (4.7)
- 3
- (1.4)
- 7
- (4)
Using data from the same studies, the number (%) of patients experiencing hypotension is displayed by patient age, drug and concentration in Table 5. In Table 6, the adverse events for ropivacaine hydrochloride are broken down by gender.
Table 5 Effects of Age on Hypotension (Epidural Administration)
Total N: Ropivacaine Hydrochloride = 760, Bupivacaine = 410
- AGE
- Ropivacaine Hydrochloride
- Bupivacaine
5 mg/mL
- 7.5 mg/mL
- 10 mg/mL
- 5 mg/mL
- 7.5 mg/mL
N
- (%)
- N
- (%)
- N
- (%)
- N
- (%)
- N
- (%)
- <65
- 68
- (32.2)
- 99
- (43.2)
- 87
- (51.5)
- 64
- (33.5)
- 73
- (48.3)
- ≥65
- 31
- (68.9)
- 47
- (69.1)
- 26
- (68.4)
- 27
- (60)
- 16
- (69.6)
Table 6 Most Common Adverse Events by Gender (Epidural Administration)
Total N: Females = 405, Males = 355
Adverse Reaction
Female
Male
N
(%)
N
(%)
hypotension
220
(54.3)
138
(38.9)
nausea
119
(29.4)
23
(6.5)
bradycardia
65
(16)
56
(15.8)
vomiting
59
(14.6)
8
(2.3)
back pain
41
(10.1)
23
(6.5)
headache
33
(8.1)
17
(4.8)
chills
18
(4.4)
5
(1.4)
fever
16
(4)
3
(0.8)
pruritus
16
(4)
1
(0.3)
pain
12
(3)
4
(1.1)
urinary retention
11
(2.7)
7
(2)
dizziness
9
(2.2)
4
(1.1)
hypoesthesia
8
(2)
2
(0.6)
paresthesia
8
(2)
10
(2.8)
Systemic Reactions
The most commonly encountered acute adverse experiences that demand immediate countermeasures are related to the central nervous system and the cardiovascular system. These adverse experiences are generally dose-related and due to high plasma levels that may result from overdosage, rapid absorption from the injection site, diminished tolerance or from unintentional intravascular injection of the local anesthetic solution. In addition to systemic dose-related toxicity, unintentional subarachnoid injection of drug during the intended performance of lumbar epidural block or nerve blocks near the vertebral column (especially in the head and neck region) may result in underventilation or apnea ("Total or High Spinal"). Also, hypotension due to loss of sympathetic tone and respiratory paralysis or underventilation due to cephalad extension of the motor level of anesthesia may occur. This may lead to secondary cardiac arrest if untreated. Factors influencing plasma protein binding, such as acidosis, systemic diseases that alter protein production or competition with other drugs for protein binding sites, may diminish individual tolerance.
Epidural administration of ropivacaine hydrochloride has, in some cases, as with other local anesthetics, been associated with transient increases in temperature to > 38.5°C. This occurred more frequently at doses of ropivacaine hydrochloride > 16 mg/h.
Neurologic Reactions
These are characterized by excitation and/or depression. Restlessness, anxiety, dizziness, tinnitus, blurred vision or tremors may occur, possibly proceeding to convulsions. However, excitement may be transient or absent, with depression being the first manifestation of an adverse reaction. This may quickly be followed by drowsiness merging into unconsciousness and respiratory arrest. Other central nervous system effects may be nausea, vomiting, chills, and constriction of the pupils.
The incidence of convulsions associated with the use of local anesthetics varies with the route of administration and the total dose administered. In a survey of studies of epidural anesthesia, overt toxicity progressing to convulsions occurred in approximately 0.1% of local anesthetic administrations.
The incidence of adverse neurological reactions associated with the use of local anesthetics may be related to the total dose and concentration of local anesthetic administered and are also dependent upon the particular drug used, the route of administration, and the physical status of the patient. Many of these observations may be related to local anesthetic techniques, with or without a contribution from the drug. During lumbar epidural block, occasional unintentional penetration of the subarachnoid space by the catheter or needle may occur. Subsequent adverse effects may depend partially on the amount of drug administered intrathecally as well as the physiological and physical effects of a dural puncture. These observations may include spinal block of varying magnitude (including high or total spinal block), hypotension secondary to spinal block, urinary retention, loss of bladder and bowel control (fecal and urinary incontinence), and loss of perineal sensation and sexual function. Signs and symptoms of subarachnoid block typically start within 2 to 3 minutes of injection. Doses of 15 and 22.5 mg of ropivacaine hydrochloride resulted in sensory levels as high as T5 and T4, respectively. Analgesia started in the sacral dermatomes in 2 to 3 minutes and extended to the T10 level in 10 to 13 minutes and lasted for approximately 2 hours. Other neurological effects following unintentional subarachnoid administration during epidural anesthesia may include persistent anesthesia, paresthesia, weakness, paralysis of the lower extremities, and loss of sphincter control; all of which may have slow, incomplete or no recovery. Headache, septic meningitis, meningismus, slowing of labor, increased incidence of forceps delivery, or cranial nerve palsies due to traction on nerves from loss of cerebrospinal fluid have been reported [see Dosage and Administration (2.1)]. A high spinal is characterized by paralysis of the arms, loss of consciousness, respiratory paralysis and bradycardia.
Cardiovascular System Reactions
High doses or unintentional intravascular injection may lead to high plasma levels and related depression of the myocardium, decreased cardiac output, heart block, hypotension, bradycardia, ventricular arrhythmias, including ventricular tachycardia and ventricular fibrillation, and possibly cardiac arrest [see Warnings and Precautions (5.2) and Overdosage (10)].
Allergic Reactions
Allergic type reactions are rare and may occur as a result of sensitivity to the local anesthetic [see Warnings and Precautions (5.1)]. These reactions are characterized by signs such as urticaria, pruritus, erythema, angioneurotic edema (including laryngeal edema), tachycardia, sneezing, nausea, vomiting, dizziness, syncope, excessive sweating, elevated temperature, and possibly, anaphylactoid symptomatology (including severe hypotension). Cross-sensitivity among members of the amide-type local anesthetic group has been reported. The usefulness of screening for sensitivity has not been definitively established.
Close -
7 DRUG INTERACTIONS Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics ...
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics [see Warnings and Precautions (5.4)]:
Examples of Drugs Associated with Methemoglobinemia
Class
Examples
Nitrates/Nitrites
nitric oxide, nitroglycerin, nitroprusside, nitrous oxide
Local anesthetics
articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine
Antineoplastic agents
cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase
Antibiotics
dapsone, nitrofurantoin, para-aminosalicylic acid, sulfonamides
Antimalarials
chloroquine, primaquine
Anticonvulsants
Phenobarbital, phenytoin, sodium valproate
Other drugs
acetaminophen, metoclopramide, quinine, sulfasalazine
Ropivacaine hydrochloride should be used with caution in patients receiving other local anesthetics or agents structurally related to amide-type local anesthetics, since the toxic effects of these drugs are additive. Cytochrome P4501A2 is involved in the formation of 3-hydroxy ropivacaine, the major metabolite. In vivo, the plasma clearance of ropivacaine was reduced by 70% during coadministration of fluvoxamine (25 mg bid for 2 days), a selective and potent CYP1A2 inhibitor. Thus strong inhibitors of cytochrome P4501A2, such as fluvoxamine, given concomitantly during administration of ropivacaine hydrochloride, can interact with ropivacaine hydrochloride leading to increased ropivacaine plasma levels. Caution should be exercised when CYP1A2 inhibitors are coadministered. Possible interactions with drugs known to be metabolized by CYP1A2 via competitive inhibition such as theophylline and imipramine may also occur. Coadministration of a selective and potent inhibitor of CYP3A4, ketoconazole (100 mg bid for 2 days with ropivacaine infusion administered 1 hour after ketoconazole) caused a 15% reduction in in vivo plasma clearance of ropivacaine.
Specific trials studying the interaction between ropivacaine and class III antiarrhythmic drugs (e.g., amiodarone) have not been performed, but caution is advised [see Warnings and Precautions (5.13)].
Close -
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no available human data on use of ropivacaine hydrochloride injection in pregnant women to evaluate a drug-associated risk of major birth defects ...
8.1 Pregnancy
Risk Summary
There are no available human data on use of ropivacaine hydrochloride injection in pregnant women to evaluate a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Local anesthetics may cause varying degrees of toxicity to the mother and fetus and adverse reactions include alterations of the central nervous system, peripheral vascular tone, and cardiac function (see Clinical Considerations). No teratogenicity was observed at doses up to 0.3 times the maximum recommended human dose of 770 mg/24 hours for epidural use, and equal to the MRHD of 250 mg for nerve block use, based on body surface area (BSA) comparisons and a 60 kg human weight (see Animal data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U. S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Labor or Delivery
Local anesthetics, including ropivacaine, rapidly cross the placenta, and when used for epidural block can cause varying degrees of maternal, fetal, and neonatal toxicity [see Clinical Pharmacology (12)]. The incidence and degree of toxicity depend upon the procedure performed, the type and amount of drug used, and the technique of drug administration. Adverse reactions in the parturient, fetus and neonate involve alterations of the central nervous system, peripheral vascular tone and cardiac function.
Maternal Adverse reactions
Maternal hypotension has resulted from regional anesthesia. Local anesthetics produce vasodilation by blocking sympathetic nerves. Therefore, during treatment of systemic toxicity, maternal hypotension or fetal bradycardia following regional block, the parturient should be maintained in the left lateral decubitus position if possible, or manual displacement of the uterus off the great vessels be accomplished. Elevating the patient's legs will also help prevent decreases in blood pressure. The fetal heart rate also should be monitored continuously, and electronic fetal monitoring is highly advisable.
Data
Animal data
No malformations were reported in embryo-fetal development toxicity studies conducted in pregnant New Zealand white rabbits and Sprague-Dawley rats. During gestation days 6 to 18, rabbits received daily subcutaneous doses of ropivacaine at 1.3, 4.2, or 13 mg/kg/day (equivalent to 0.03, 0.10, and 0.33 times the maximum recommended human dose (MRHD) of 770 mg/24 hours, respectively, and 0.10, 0.32, and 1.0 times the MRHD of 250 mg for nerve block use, respectively based on body surface area (BSA) comparisons and a 60 kg human weight). Rats received daily subcutaneous doses of 5.3, 11, and 26 mg/kg/day (equivalent to 0.07, 0.14, and 0.33 times the MRHD for epidural use, respectively, and 0.21, 0.43, and 1.0 times the MRHD for nerve block use, respectively, based on BSA comparisons) during GD 6 to 15.
No treatment-related effects on late fetal development, parturition, litter size, lactation, neonatal viability, or growth of the offspring were reported in a prenatal and postnatal reproductive and development toxicity study; however functional endpoints were not evaluated. Female rats were dosed daily subcutaneously from GD 15 to Lactation Day 20 at doses of 5.3, 11, and 26 mg/kg/day (equivalent to 0.07, 0.1, and 0.3 times the MRHD for epidural use, respectively, and 0.21, 0.43, and 1.0 times the MRHD for nerve block use, respectively), with maternal toxicity exhibited at the high dose.
No adverse effects in physical developmental milestones or in behavioral tests were reported in a 2-generational reproduction study, in which rats received daily subcutaneous doses of 6.3, 12, and 23 mg/kg/day (equivalent to 0.08, 0.15, and 0.29 times the MRHD for epidural use, respectively, and 0.24, 0.45, and 0.88 times the MRHD for nerve block use, respectively, based on BSA comparisons) for 9 weeks before mating and during mating for males, and for 2 weeks before mating and during mating, pregnancy, and lactation, up to day 42 post coitus for females. Significant pup loss was observed in the high dose group during the first 3 days postpartum, from a few hours up to 3 days after delivery compared to the control group, which was considered secondary to impaired maternal care due to maternal toxicity. No differences were observed in litter parameters, or fertility, mean gestation time, or number of live births were observed between the control (saline) and treatment groups [see Carcinogenesis, Mutagenesis, Impairment of Fertility (13.1)].
8.2 Lactation
Risk Summary
One publication reported that ropivacaine is present in human milk at low levels following administration of ropivacaine in women undergoing cesarean section. No adverse reactions were reported in the infants. There is no available information on the drug’s effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ropivacaine hydrochloride and any potential adverse effects on the breastfed child from ropivacaine hydrochloride or from the underlying maternal condition.
8.4 Pediatric Use
The safety and efficacy of ropivacaine hydrochloride in pediatric patients have not been established.
8.5 Geriatric Use
Of the 2,978 subjects that were administered ropivacaine hydrochloride injection in 71 controlled and uncontrolled clinical studies, 803 patients (27%) were 65 years of age or older which includes 127 patients (4%) 75 years of age and over. Ropivacaine hydrochloride injection was found to be safe and effective in the patients in these studies. Clinical data in one published article indicate that differences in various pharmacodynamic measures were observed with increasing age. In one study, the upper level of analgesia increased with age, the maximum decrease of mean arterial pressure (MAP) declined with age during the first hour after epidural administration, and the intensity of motor blockade increased with age.
This drug and its metabolites are known to be excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Elderly patients are more likely to have decreased hepatic, renal, or cardiac function, as well as concomitant disease. Therefore, care should be taken in dose selection, starting at the low end of the dosage range, and it may be useful to monitor renal function [see Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
Because amide-type local anesthetics such as ropivacaine are metabolized by the liver, these drugs, especially repeat doses, should be used cautiously in patients with hepatic disease. Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at a greater risk of developing toxic plasma concentrations [see Warning and Precautions (5.11)].
Close8.7 Renal Impairment
This drug and its metabolites are known to be excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Therefore, care should be taken in dose selection, starting at the low end of the dosage range, and it may be useful to monitor renal function [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE Acute emergencies from local anesthetics are generally related to high plasma levels encountered, or large doses administered, during therapeutic use of local anesthetics or to unintended ...
Acute emergencies from local anesthetics are generally related to high plasma levels encountered, or large doses administered, during therapeutic use of local anesthetics or to unintended subarachnoid or intravascular injection of local anesthetic solution [see Adverse Reactions (6) and Warnings and Precautions (5.1, 5.2, 5.6)].
Close10.1 Treatment
Therapy with ropivacaine hydrochloride should be discontinued at the first sign of toxicity. No specific information is available for the treatment of toxicity with ropivacaine hydrochloride; therefore, treatment should be symptomatic and supportive. The first consideration is prevention, best accomplished by incremental injection of ropivacaine hydrochloride, careful and constant monitoring of cardiovascular and respiratory vital signs and the patient’s state of consciousness after each local anesthetic and during continuous infusion. At the first sign of change in mental status, oxygen should be administered.
The first step in the management of systemic toxic reactions, as well as underventilation or apnea due to unintentional subarachnoid injection of drug solution, consists of immediate attention to the establishment and maintenance of a patent airway and effective assisted or controlled ventilation with 100% oxygen with a delivery system capable of permitting immediate positive airway pressure by mask. Circulation should be assisted as necessary. This may prevent convulsions if they have not already occurred.
If necessary, use drugs to control convulsions. Intravenous barbiturates, anticonvulsant agents, or muscle relaxants should only be administered by those familiar with their use. Immediately after the institution of these ventilatory measures, the adequacy of the circulation should be evaluated. Supportive treatment of circulatory depression may require administration of intravenous fluids, and, when appropriate, a vasopressor dictated by the clinical situation (such as ephedrine or epinephrine to enhance myocardial contractile force).
Should cardiac arrest occur, prolonged resuscitative efforts may be required to improve the probability of a successful outcome.
The mean dosages of ropivacaine producing seizures, after intravenous infusion in dogs, nonpregnant and pregnant sheep were 4.9, 6.1 and 5.9 mg/kg, respectively. These doses were associated with peak arterial total plasma concentrations of 11.4, 4.3 and 5 mcg/mL, respectively.
In human volunteers given intravenous ropivacaine hydrochloride, the mean (min-max) maximum tolerated total and free arterial plasma concentrations were 4.3 (3.4 to 5.3) and 0.6 (0.3 to 0.9) mcg/mL respectively, at which time moderate CNS symptoms (muscle twitching) were noted.
Clinical data from patients experiencing local anesthetic induced convulsions demonstrated rapid development of hypoxia, hypercarbia and acidosis within a minute of the onset of convulsions. These observations suggest that oxygen consumption and carbon dioxide production are greatly increased during local anesthetic convulsions and emphasize the importance of immediate and effective ventilation with oxygen which may avoid cardiac arrest.
If difficulty is encountered in the maintenance of a patent airway or if prolonged ventilatory support (assisted or controlled) is indicated, endotracheal intubation, employing drugs and techniques familiar to the clinician, may be indicated after initial administration of oxygen by mask.
The supine position is dangerous in pregnant women at term because of aortocaval compression by the gravid uterus. Therefore, during treatment of systemic toxicity, maternal hypotension or fetal bradycardia following regional block, the parturient should be maintained in the left lateral decubitus position if possible, or manual displacement of the uterus off the great vessels should be accomplished. Resuscitation of obstetrical patients may take longer than resuscitation of non-pregnant patients and closed-chest cardiac compression may be ineffective. Rapid delivery of the fetus may improve the response to resuscitative efforts.
-
11 DESCRIPTION Ropivacaine hydrochloride injection, USP is a sterile, isotonic solution that contains ropivacaine hydrochloride as the active pharmaceutical ingredient. Ropivacaine hydrochloride is a member of ...
Ropivacaine hydrochloride injection, USP is a sterile, isotonic solution that contains ropivacaine hydrochloride as the active pharmaceutical ingredient. Ropivacaine hydrochloride is a member of the amino amide class of local anesthetics. Ropivacaine hydrochloride injection, USP is administered parenterally by for infiltration, epidural, and nerve block.
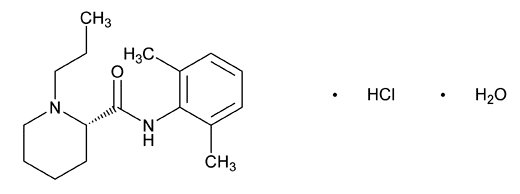
Ropivacaine hydrochloride is chemically described as S-(-)-1-propyl-2',6'-pipecoloxylidide hydrochloride monohydrate. The drug substance is a white crystalline powder, with the following structural formula:
C17H26N2O•HCl•H2O M.W. 328.89
At 25°C ropivacaine hydrochloride has a solubility of 53.8 mg/mL in water, a distribution ratio between n-octanol and phosphate buffer at pH 7.4 of 14:1 and a pKa of 8.07 in 0.1 M KCl solution. The pKa of ropivacaine is approximately the same as bupivacaine (8.1) and is similar to that of mepivacaine (7.7). However, ropivacaine has an intermediate degree of lipid solubility compared to bupivacaine and mepivacaine.
Ropivacaine hydrochloride injection, USP is a clear, colorless, and preservative-free solution. Each mL contains 2.1 mg ropivacaine hydrochloride monohydrate (equivalent to 2 mg of ropivacaine hydrochloride anhydrous), and 8.6 mg of sodium chloride; and sodium hydroxide and hydrochloric acid as pH adjusters, in water for injection. The pH is adjusted between 4.0 to 6.0 with. The specific gravity of ropivacaine hydrochloride injection, USP solutions range from 1.002 to 1.009 at 25°C.
Close -
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Ropivacaine is a member of the amino amide class of local anesthetics and is supplied as the pure S-(-)-enantiomer. Local anesthetics block the generation and the ...
12.1 Mechanism of Action
Ropivacaine is a member of the amino amide class of local anesthetics and is supplied as the pure S-(-)-enantiomer. Local anesthetics block the generation and the conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential. In general, the progression of anesthesia is related to the diameter, myelination and conduction velocity of affected nerve fibers. Clinically, the order of loss of nerve function is as follows: (1) pain, (2) temperature, (3) touch, (4) proprioception, and (5) skeletal muscle tone.
12.2 Pharmacodynamics
Studies in humans have demonstrated that, unlike most other local anesthetics, the presence of epinephrine has no major effect on either the time of onset or the duration of action of ropivacaine. Likewise, addition of epinephrine to ropivacaine has no effect on limiting systemic absorption of ropivacaine.
Systemic absorption of local anesthetics can produce effects on the central nervous and cardiovascular systems. At blood concentrations achieved with therapeutic doses, changes in cardiac conduction, excitability, refractoriness, contractility, and peripheral vascular resistance have been reported. Toxic blood concentrations depress cardiac conduction and excitability, which may lead to atrioventricular block, ventricular arrhythmias and to cardiac arrest, sometimes resulting in fatalities. In addition, myocardial contractility is depressed and peripheral vasodilation occurs, leading to decreased cardiac output and arterial blood pressure.
Following systemic absorption, local anesthetics can produce central nervous system stimulation, depression or both. Apparent central stimulation is usually manifested as restlessness, tremors and shivering, progressing to convulsions, followed by depression and coma, progressing ultimately to respiratory arrest. However, the local anesthetics have a primary depressant effect on the medulla and on higher centers. The depressed stage may occur without a prior excited stage.
In 2 clinical pharmacology studies (total n=24) ropivacaine and bupivacaine were infused (10 mg/min) in human volunteers until the appearance of CNS symptoms, e.g., visual or hearing disturbances, perioral numbness, tingling and others. Similar symptoms were seen with both drugs. In 1 study, the mean ± SD maximum tolerated intravenous dose of ropivacaine infused (124 ± 38 mg) was significantly higher than that of bupivacaine (99 ± 30 mg) while in the other study the doses were not different (115 ± 29 mg of ropivacaine and 103 ± 30 mg of bupivacaine). In the latter study, the number of subjects reporting each symptom was similar for both drugs with the exception of muscle twitching which was reported by more subjects with bupivacaine than ropivacaine at comparable intravenous doses. At the end of the infusion, ropivacaine in both studies caused significantly less depression of cardiac conductivity (less QRS widening) than bupivacaine. Ropivacaine and bupivacaine caused evidence of depression of cardiac contractility, but there were no changes in cardiac output.
Clinical data in one published article indicate that differences in various pharmacodynamic measures were observed with increasing age. In one study, the upper level of analgesia increased with age, the maximum decrease of mean arterial pressure (MAP) declined with age during the first hour after epidural administration, and the intensity of motor blockade increased with age. However, no pharmacokinetic differences were observed between elderly and younger patients.
In non-clinical pharmacology studies comparing ropivacaine and bupivacaine in several animal species, the cardiac toxicity of ropivacaine was less than that of bupivacaine, although both were considerably more toxic than lidocaine. Arrhythmogenic and cardio-depressant effects were seen in animals at significantly higher doses of ropivacaine than bupivacaine. The incidence of successful resuscitation was not significantly different between the ropivacaine and bupivacaine groups.
Close12.3 Pharmacokinetics
Absorption
The systemic concentration of ropivacaine is dependent on the total dose and concentration of drug administered, the route of administration, the patient's hemodynamic/circulatory condition, and the vascularity of the administration site.
From the epidural space, ropivacaine shows complete and biphasic absorption. The half-lives of the 2 phases, (mean ± SD) are 14 ± 7 minutes and 4.2 ± 0.9 h, respectively. The slow absorption is the rate limiting factor in the elimination of ropivacaine that explains why the terminal half-life is longer after epidural than after intravenous administration. Ropivacaine shows dose-proportionality up to the highest intravenous dose studied, 80 mg, corresponding to a mean ± SD peak plasma concentration of 1.9 ± 0.3 mcg/mL.
Table 7 Pharmacokinetic (Plasma Concentration-Time) Data From Clinical Trials
Route
Epidural Infusion*
Epidural
Infusion*
Epidural
Block†
Epidural
Block†
Plexus
Block‡
IV
Infusion§
Dose (mg)
1493 ± 10
2075 ± 206
1217 ± 277
150
187.5
300
40
N
12
12
11
8
8
10
12
Cmax (mg/L)
2.4 ± 1¶
2.8 ± 0.5¶
2.3 ± 1.1¶
1.1 ± 0.2
1.6 ± 0.6
2.3 ± 0.8
1.2 ± 0.2#
Tmax (min)
n/a♠
n/a
n/a
43 ± 14
34 ± 9
54 ± 22
n/a
AUC0-
(mg.h/L)
135.5 ± 50
145 ± 34
161 ± 90
7.2 ± 2
11.3 ± 4
13 ± 3.3
1.8 ± 0.6
CL (L/h)
11.03
13.7
n/a
5.5 ± 2
5 ± 2.6
n/a
21.2 ± 7
t1/2 (hr)♥
5 ± 2.5
5.7 ± 3
6 ± 3
5.7 ± 2
7.1 ± 3
6.8 ± 3.2
1.9 ± 0.5
* Continuous 72 hour epidural infusion after an epidural block with 5 or 10 mg/mL.
† Epidural anesthesia with 7.5 mg/mL (0.75%) for cesarean delivery.
‡ Brachial plexus block with 7.5 mg/mL (0.75%) ropivacaine.
§ 20 minute IV infusion to volunteers (40 mg).
¶ Cmax measured at the end of infusion (i.e., at 72 hr).
# Cmax measured at the end of infusion (i.e., at 20 minutes).
♠ n/a=not applicable
♥ t½ is the true terminal elimination half-life. On the other hand, t½ follows absorption dependent elimination (flip-flop) after non-intravenous administration.
In some patients after a 300 mg dose for brachial plexus block, free plasma concentrations of ropivacaine may approach the threshold for CNS toxicity [see Warnings and Precautions (5.7)]. At a dose of greater than 300 mg, for local infiltration, the terminal half-life may be longer (>30 hours).
Distribution
After intravascular infusion, ropivacaine has a steady-state volume of distribution of 41 ± 7 liters. Ropivacaine is 94% protein bound, mainly to α1-acid glycoprotein. An increase in total plasma concentrations during continuous epidural infusion has been observed, related to a postoperative increase of α1-acid glycoprotein. Variations in unbound, i.e., pharmacologically active, concentrations have been less than in total plasma concentration. Ropivacaine readily crosses the placenta and equilibrium in regard to unbound concentration will be rapidly reached [see Warnings and Precautions (5) and Use in Specific Population (8.1)].
Metabolism
Ropivacaine is extensively metabolized in the liver, predominantly by aromatic hydroxylation mediated by cytochrome P4501A to 3-hydroxy ropivacaine. After a single IV dose approximately 37% of the total dose is excreted in the urine as both free and conjugated 3-hydroxy ropivacaine. Low concentrations of 3-hydroxy ropivacaine have been found in the plasma. Urinary excretion of the 4 hydroxy ropivacaine, and both the 3-hydroxy N-de-alkylated (3-OH-PPX) and 4-hydroxy N-de-alkylated (4-OH-PPX) metabolites account for less than 3% of the dose. An additional metabolite, 2-hydroxy-methylropivacaine, has been identified but not quantified in the urine. The N-de-alkylated metabolite of ropivacaine (PPX) and 3-OH-ropivacaine are the major metabolites excreted in the urine during epidural infusion. Total PPX concentration in the plasma was about half as that of total ropivacaine; however, mean unbound concentrations of PPX were about 7 to 9 times higher than that of unbound ropivacaine following continuous epidural infusion up to 72 hours. Unbound PPX, 3-hydroxy and 4-hydroxy ropivacaine, have a pharmacological activity in animal models less than that of ropivacaine. There is no evidence of in vivo racemization in urine of ropivacaine.
Elimination
The kidney is the main excretory organ for most local anesthetic metabolites. In total, 86% of the ropivacaine dose is excreted in the urine after intravenous administration of which only 1% relates to unchanged drug. After intravenous administration ropivacaine has a mean ± SD total plasma clearance of 387 ± 107 mL/min, an unbound plasma clearance of 7.2 ± 1.6 L/min, and a renal clearance of 1 mL/min. The mean ± SD terminal half-life is 1.8 ± 0.7 h after intravascular administration and 4.2 ± 1 h after epidural administration.
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term studies in animals to evaluate the carcinogenic potential of ropivacaine have not been ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals to evaluate the carcinogenic potential of ropivacaine have not been conducted.
Mutagenesis
Weak mutagenic activity was seen in the mouse lymphoma test. However, ropivacaine was negative in an in vitro Ames assay and an in vivo mouse micronucleus assay.
Impairment of Fertility
No adverse effects on fertility or early embryonic development were reported in a 2-generational reproduction study in which female rats (F0) were administered subcutaneous doses of 6.3, 12, and 23 mg/kg/day (equivalent to 0.08, 0.15, and 0.29 times the maximum recommended human dose (MRHD) of 770 mg/24 hours for epidural use, respectively, and 0.24, 0.45, and 0.88 times the MRHD of 250 mg for nerve block use, respectively, based on BSA comparisons and a 60 kg human) throughout the mating period and pregnancy, partus, and lactation.
Close13.2 Animal Toxicology and/or Pharmacology
The mean dosages of ropivacaine producing seizures, after intravenous infusion in dogs, nonpregnant and pregnant sheep were 4.9, 6.1 and 5.9 mg/kg (HED: 5.3, 6.6, and 6.4 mg/kg, based on 75 kg sheep weight and 60 kg human weight), respectively. These doses were associated with peak arterial total plasma concentrations of 11.4, 4.3 and 5 mcg/mL, respectively.
-
14 CLINICAL STUDIES Ropivacaine was studied as a local anesthetic both for surgical anesthesia and for acute pain management [see Dosage and Administration (2)]. The onset, depth and duration of sensory block are, in ...
Ropivacaine was studied as a local anesthetic both for surgical anesthesia and for acute pain management [see Dosage and Administration (2)].
The onset, depth and duration of sensory block are, in general, similar to bupivacaine. However, the depth and duration of motor block, in general, are less than that with bupivacaine.
14.1 Epidural Administration in Surgery
There were 25 clinical studies performed in 900 patients to evaluate ropivacaine hydrochloride epidural injection for general surgery. Ropivacaine hydrochloride was used in doses ranging from 75 to 250 mg. In doses of 100 to 200 mg, the median (1st to 3rd quartile) onset time to achieve a T10 sensory block was 10 (5 to 13) minutes and the median (1st to 3rd quartile) duration at the T10 level was 4 (3 to 5) hours [see Dosage and Administration (2.2)]. Higher doses produced a more profound block with a greater duration of effect.
14.2 Epidural Administration in Cesarean Section
A total of 12 studies were performed with epidural administration of ropivacaine hydrochloride for cesarean section. Eight of these studies involved 218 patients using the concentration of 5 mg/mL (0.5%) in doses up to 150 mg. Median onset measured at T6 ranged from 11 to 26 minutes. Median duration of sensory block at T6 ranged from 1.7 to 3.2 h, and duration of motor block ranged from 1.4 to 2.9 h. Ropivacaine hydrochloride provided adequate muscle relaxation for surgery in all cases.
In addition, 4 active controlled studies for cesarean section were performed in 264 patients at a concentration of 7.5 mg/mL (0.75%) in doses up to 187.5 mg. Median onset measured at T6 ranged from 4 to 15 minutes. Seventy-seven to 96% of ropivacaine hydrochloride-exposed patients reported no pain at delivery. Some patients received other anesthetic, analgesic, or sedative modalities during the course of the operative procedure.
14.3 Epidural Administration in Labor and Delivery
A total of 9 double-blind clinical studies, involving 240 patients were performed to evaluate ropivacaine hydrochloride for epidural block for management of labor pain. When administered in doses up to 278 mg as intermittent injections or as a continuous infusion, ropivacaine hydrochloride produced adequate pain relief.
A prospective meta-analysis on 6 of these studies provided detailed evaluation of the delivered newborns and showed no difference in clinical outcomes compared to bupivacaine. There were significantly fewer instrumental deliveries in mothers receiving ropivacaine as compared to bupivacaine.
Table 8 Labor and Delivery Meta-analysis: Mode of Delivery
Delivery Mode
Ropivacaine Hydrochloride
n = 199
Bupivacaine
n = 188
N
%
n
%
Spontaneous Vertex
116
58
92
49
Vacuum Extractor
26
33
}27*
}40
Forceps
28
42
Cesarean Section
29
15
21
11
*p=0.004 versus bupivacaine
14.4 Epidural Administration in Postoperative Pain Management
There were 8 clinical studies performed in 382 patients to evaluate ropivacaine hydrochloride 2 mg/mL (0.2%) for postoperative pain management after upper and lower abdominal surgery and after orthopedic surgery. The studies utilized intravascular morphine via PCA as a rescue medication and quantified as an efficacy variable.
Epidural anesthesia with ropivacaine hydrochloride 5 mg/mL, (0.5%) was used intraoperatively for each of these procedures prior to initiation of postoperative ropivacaine hydrochloride. The incidence and intensity of the motor block were dependent on the dose rate of ropivacaine hydrochloride and the site of injection. Cumulative doses of up to 770 mg of ropivacaine were administered over 24 hours (intraoperative block plus postoperative continuous infusion). The overall quality of pain relief, as judged by the patients, in the ropivacaine groups was rated as good or excellent (73% to 100%). The frequency of motor block was greatest at 4 hours and decreased during the infusion period in all groups. At least 80% of patients in the upper and lower abdominal studies and 42% in the orthopedic studies had no motor block at the end of the 21-hour infusion period. Sensory block was also dose rate dependent and a decrease in spread was observed during the infusion period.
A double-blind, randomized, clinical trial compared lumbar epidural infusion of ropivacaine hydrochloride (n=26) and bupivacaine (n=26) at 2 mg/mL (8 mL/h), for 24 hours after knee replacement. In this study, the pain scores were higher in the ropivacaine hydrochloride group, but the incidence and the intensity of motor block were lower.
Continuous epidural infusion of ropivacaine hydrochloride 2 mg/mL (0.2%) during up to 72 hours for postoperative pain management after major abdominal surgery was studied in 2 multicenter, double-blind studies. A total of 391 patients received a low thoracic epidural catheter, and ropivacaine hydrochloride 7.5 mg/L (0.75%) was given for surgery, in combination with GA. Postoperatively, ropivacaine hydrochloride 2 mg/mL (0.2%), 4 to 14 mL/h, alone or with fentanyl 1, 2, or 4 mcg/mL was infused through the epidural catheter and adjusted according to the patient’s needs. These studies support the use of ropivacaine hydrochloride 2 mg/mL (0.2%) for epidural infusion at 6 to 14 mL/h (12 to 28 mg) for up to 72 hours and demonstrated adequate analgesia with only slight and nonprogressive motor block in cases of moderate to severe postoperative pain.
Clinical studies with 2 mg/mL (0.2%) ropivacaine hydrochloride have demonstrated that infusion rates of 6 to 14 mL (12 to 28 mg) per hour provide adequate analgesia with nonprogressive motor block in cases of moderate to severe postoperative pain. In these studies, this technique resulted in a significant reduction in patients’ morphine rescue dose requirement. Clinical experience supports the use of ropivacaine hydrochloride epidural infusions for up to 72 hours.
14.5 Peripheral Nerve Block
Ropivacaine hydrochloride, 5 mg/mL (0.5%), was evaluated for its ability to provide anesthesia for surgery using the techniques of Peripheral Nerve Block. There were 13 studies performed including a series of 4 pharmacodynamic and pharmacokinetic studies performed on minor nerve blocks. From these, 235 ropivacaine hydrochloride-treated patients were evaluable for efficacy. Ropivacaine hydrochloride was used in doses up to 275 mg. When used for brachial plexus block, onset depended on technique used. Supraclavicular blocks were consistently more successful than axillary blocks. The median onset of sensory block (anesthesia) produced by ropivacaine 0.5% via axillary block ranged from 10 minutes (medial brachial cutaneous nerve) to 45 minutes (musculocutaneous nerve). Median duration ranged from 3.7 hours (medial brachial cutaneous nerve) to 8.7 hours (ulnar nerve). The 5 mg/mL (0.5%) ropivacaine hydrochloride solution gave success rates from 56% to 86% for axillary blocks, compared with 92% for supraclavicular blocks.
In addition, ropivacaine hydrochloride, 7.5 mg/mL (0.75%), was evaluated in 99 ropivacaine hydrochloride-treated patients, in 2 double-blind studies, performed to provide anesthesia for surgery using the techniques of Brachial Plexus Block. Ropivacaine hydrochloride 7.5 mg/mL was compared to bupivacaine 5 mg/mL. In 1 study, patients underwent axillary brachial plexus block using injections of 40 mL (300 mg) of ropivacaine hydrochloride, 7.5 mg/mL (0.75%) or 40 mL injections of bupivacaine, 5 mg/mL (200 mg). In a second study, patients underwent subclavian perivascular brachial plexus block using 30 mL (225 mg) of ropivacaine hydrochloride, 7.5 mg/mL (0.75%) or 30 mL of bupivacaine 5 mg/mL (150 mg). There was no significant difference between the ropivacaine hydrochloride and bupivacaine groups in either study with regard to onset of anesthesia, duration of sensory blockade, or duration of anesthesia.
The median duration of anesthesia varied between 11.4 and 14.4 hours with both techniques. In one study, using the axillary technique, the quality of analgesia and muscle relaxation in the ropivacaine hydrochloride group was judged to be significantly superior to bupivacaine by both investigator and surgeon. However, using the subclavian perivascular technique, no statistically significant difference was found in the quality of analgesia and muscle relaxation as judged by both the investigator and surgeon. The use of ropivacaine hydrochloride 7.5 mg/mL for block of the brachial plexus via either the subclavian perivascular approach using 30 mL (225 mg) or via the axillary approach using 40 mL (300 mg) both provided effective and reliable anesthesia.
Close14.6 Local Infiltration
A total of 7 clinical studies were performed to evaluate the local infiltration of ropivacaine hydrochloride to produce anesthesia for surgery and analgesia in postoperative pain management. In these studies 297 patients who received ropivacaine hydrochloride in doses up to 200 mg (concentrations up to 5 mg/mL, 0.5%) were evaluable for efficacy. With infiltration of 100 to 200 mg ropivacaine hydrochloride, the time to first request for analgesic was 2 to 6 hours. When compared to placebo, ropivacaine hydrochloride produced lower pain scores and a reduction of analgesic consumption.
-
16 How Supplied/Storage and Handling Ropivacaine Hydrochloride Injection, USP is a clear colorless, and preservative-free solution, available in single-dose containers in 2 mg/mL (0.2%) concentration. Storage - Solutions should be ...
Ropivacaine Hydrochloride Injection, USP is a clear colorless, and preservative-free solution, available in single-dose containers in 2 mg/mL (0.2%) concentration.
Storage
Solutions should be stored at 20ºC to 25°C (68ºF to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Ropivacaine Hydrochloride Injection, USP single-dose, ready-to-use, polypropylene flexible bag. The flexible bag container is not made with natural rubber latex or polyvinyl chloride (PVC), Non-DEHP.
Unit of Sale
Strength
Each
NDC 67457-798-99
Unit of 10
(0.2 %) 200 mg per 100 mL (2 mg/mL)
NDC 67457-798-10
100 mL single-dose, ready-to-use, polypropylene flexible bag
NDC 67457-798-98
Unit of 24
(0.2 %) 200 mg per 100 mL (2 mg/mL)
NDC 67457-798-10
100 mL single-dose, ready-to-use, polypropylene flexible bag
NDC 67457-799-99
Unit of 10
(0.2 %) 400 mg per 200 mL (2 mg/mL)
NDC 67457-799-20
200 mL single-dose, ready-to-use, polypropylene flexible bag
NDC 67457-799-98
Unit of 24
(0.2 %) 400 mg per 200 mL (2 mg/mL)
NDC 67457-799-20
200 mL single-dose, ready-to-use, polypropylene flexible bag
Ropivacaine Hydrochloride Injection, USP container closure is not made with natural rubber latex.
Close -
17 PATIENT COUNSELING INFORMATION 17.1 Information for Patients and Caregivers - When appropriate, patients should be informed in advance that they may experience temporary loss of sensation and motor activity in the ...Close
17.1 Information for Patients and Caregivers
When appropriate, patients should be informed in advance that they may experience temporary loss of sensation and motor activity in the anesthetized part of the body following proper administration of lumbar epidural anesthesia. Also, when appropriate, the physician should discuss other information including adverse reactions in the ropivacaine hydrochloride package insert.
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
Manufactured for:
Mylan Institutional LLC
Morgantown, WV 26505 U.S.A.
Manufactured by:
Mylan Laboratories Limited
Bangalore, India
DECEMBER 2023
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 67457-798-10 - 100 mL - Ropivacaine Hydrochloride Injection, USP - 0.2% 200 mg/100 mL (2 mg/mL) For Infiltration, Nerve Block, and Epidural Administration Only. For continuous infiltration or ...
NDC 67457-798-10
100 mL
Ropivacaine Hydrochloride Injection, USP
0.2%
200 mg/100 mL (2 mg/mL)
For Infiltration, Nerve Block, and Epidural Administration Only.
For continuous infiltration or epidural infusion only
Not for Intravenous Administration.
Discard unused portion.
One 100 mL Infusion bag.
Rx only
Mylan
Single-Dose Only
Close -
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 67457-799-20 - 200 mL - Ropivacaine Hydrochloride Injection, USP - 0.2% 400 mg/200 mL (2 mg/mL) For Infiltration, Nerve Block, and Epidural Administration Only. For continuous infiltration or ...
NDC 67457-799-20
200 mL
Ropivacaine Hydrochloride Injection, USP
0.2%
400 mg/200 mL (2 mg/mL)
For Infiltration, Nerve Block, and Epidural Administration Only.
For continuous infiltration or epidural infusion only.
Not for Intravenous Administration.
Discard unused portion.
One 200 mL Infusion Bag
Rx only
Mylan
Single-Dose Only
Close -
INGREDIENTS AND APPEARANCEProduct Information
ROPIVACAINE HYDROCHLORIDE ropivacaine hydrochloride injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:67457-798 Route of Administration INFILTRATION, PERINEURAL, EPIDURAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ROPIVACAINE HYDROCHLORIDE (UNII: V910P86109) (ROPIVACAINE - UNII:7IO5LYA57N) ROPIVACAINE HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.6 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67457-798-99 10 in 1 CARTON 03/18/2024 1 NDC:67457-798-10 1 in 1 POUCH 1 100 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC:67457-798-98 24 in 1 CARTON 01/01/2030 2 1 in 1 POUCH 2 100 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206091 03/18/2024 ROPIVACAINE HYDROCHLORIDE ropivacaine hydrochloride injection, solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:67457-799 Route of Administration INFILTRATION, PERINEURAL, EPIDURAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ROPIVACAINE HYDROCHLORIDE (UNII: V910P86109) (ROPIVACAINE - UNII:7IO5LYA57N) ROPIVACAINE HYDROCHLORIDE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.6 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67457-799-99 10 in 1 CARTON 03/18/2024 1 NDC:67457-799-20 1 in 1 POUCH 1 200 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC:67457-799-98 24 in 1 CARTON 01/01/2030 2 1 in 1 POUCH 2 200 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206091 03/18/2024
CloseLabeler - Mylan Institutional LLC (790384502)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
ROPIVACAINE HYDROCHLORIDE injection, solution
Number of versions: 1
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Mar 22, 2024 | 1 (current) | download |
RxNorm
ROPIVACAINE HYDROCHLORIDE injection, solution
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 1734347 | ROPivacaine HCl 0.2 % in 100 ML Injection | PSN |
| 2 | 1734347 | 100 ML ropivacaine hydrochloride 2 MG/ML Injection | SCD |
| 3 | 1734347 | ropivacaine HCl 0.2 % per 100 ML Injection | SY |
| 4 | 1734347 | ropivacaine HCl 200 MG per 100 ML Injection | SY |
| 5 | 1734355 | ROPivacaine HCl 0.2 % in 200 ML Injection | PSN |
| 6 | 1734355 | 200 ML ropivacaine hydrochloride 2 MG/ML Injection | SCD |
| 7 | 1734355 | ropivacaine HCl 0.2 % per 200 ML Injection | SY |
| 8 | 1734355 | ropivacaine HCl 400 MG per 200 ML Injection | SY |
Get Label RSS Feed for this Drug
ROPIVACAINE HYDROCHLORIDE injection, solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=9e1d1b15-c224-4ff4-a39d-3b9cebfc8403
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
ROPIVACAINE HYDROCHLORIDE injection, solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 67457-798-10 |
| 2 | 67457-798-98 |
| 3 | 67457-798-99 |
| 4 | 67457-799-20 |
| 5 | 67457-799-98 |
| 6 | 67457-799-99 |