Label: LEUCOVORIN CALCIUM injection, powder, lyophilized, for solution
- NDC Code(s): 71288-160-10, 71288-161-20, 71288-162-30, 71288-163-30, view more
- Packager: Meitheal Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 19, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONmeitheal® Rx only
-
DESCRIPTION
Leucovorin is one of several active, chemically reduced derivatives of folic acid. It is useful as an antidote to drugs which act as folic acid antagonists. Also known as folinic acid, Citrovorum ...
-
CLINICAL PHARMACOLOGY
Leucovorin is a mixture of the diastereoisomers of the 5-formyl derivative of tetrahydrofolic acid (THF). The biologically active compound of the mixture is the (-)-l-isomer, known as Citrovorum ...
-
INDICATIONS AND USAGE
Leucovorin Calcium for Injection rescue is indicated after high dose methotrexate therapy in osteosarcoma. Leucovorin Calcium for Injection is also indicated to diminish the toxicity and ...
-
CONTRAINDICATIONS
Leucovorin calcium is improper therapy for pernicious anemia and other megaloblastic anemias secondary to the lack of vitamin B12. A hematologic remission may occur while neurologic manifestations ...
-
WARNINGS
In the treatment of accidental overdosages of folic acid antagonists, intravenous leucovorin calcium should be administered as promptly as possible. As the time interval between antifolate ...
-
PRECAUTIONS
General - Parenteral administration is preferable to oral dosing if there is a possibility that the patient may vomit and not absorb the leucovorin. Leucovorin has no effect on non-hematologic ...
-
ADVERSE REACTIONS
Allergic sensitization, including anaphylactoid reactions and urticaria, has been reported following administration of both oral and parenteral leucovorin calcium. No other adverse reactions ...
-
OVERDOSAGEExcessive amounts of leucovorin calcium may nullify the chemotherapeutic effect of folic acid antagonists.
-
DOSAGE AND ADMINISTRATION
Advanced Colorectal Cancer - Either of the following two regimens is recommended: Leucovorin calcium for injection is administered at 200 mg/m2 by slow intravenous injection over a minimum of 3 ...
-
HOW SUPPLIED
Leucovorin Calcium for Injection, USP is supplied as a sterile lyophilized powder as follows: NDCLeucovorin Calcium for Injection, USPPackage Factor - 71288-160-1050 mg Single-Dose Vial1 ...
-
REFERENCESmeitheal® Mfd. for Meitheal Pharmaceuticals - Chicago, IL 60631 (USA) ©2024 Meitheal Pharmaceuticals Inc. Mfd. by Kindos Pharmaceuticals Co., Ltd. Chengdu, China 611731 - October 2024 - LB-462-V3
-
PRINCIPAL DISPLAY PANEL – Leucovorin Calcium for Injection, USP 50 mg Vial LabelNDC 71288-160-10 - Rx Only - Leucovorin Calcium for Injection, USP - 50 mg per vial - For Intravenous or Intramuscular Use - Lyophilized - Single-Dose Vial
-
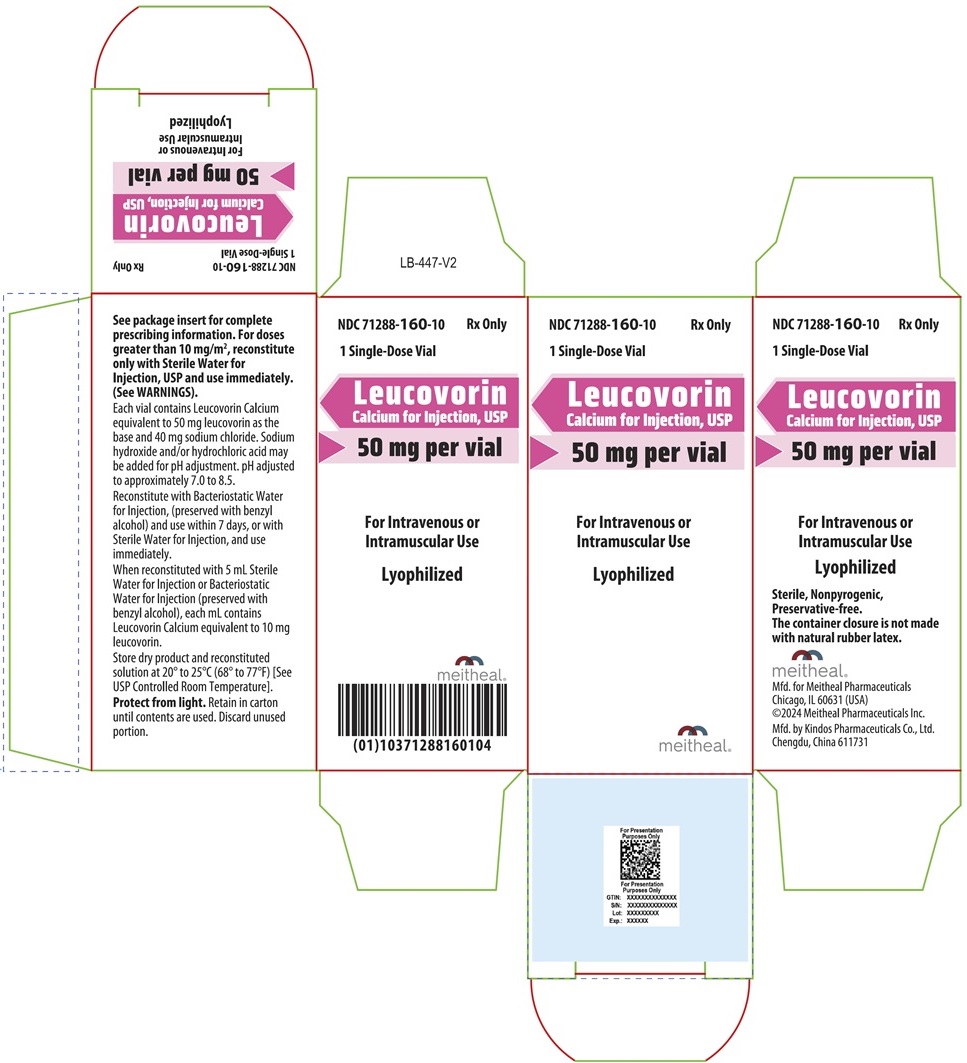
PRINCIPAL DISPLAY PANEL – Leucovorin Calcium for Injection, USP 50 mg CartonNDC 71288-160-10 - Rx Only - 1 Single-Dose Vial - Leucovorin Calcium for Injection, USP - 50 mg per vial - For Intravenous or Intramuscular Use - Lyophilized
-
PRINCIPAL DISPLAY PANEL – Leucovorin Calcium for Injection, USP 100 mg Vial LabelNDC 71288-161-20 - Rx Only - Leucovorin Calcium for Injection, USP - 100 mg per vial - For Intravenous or Intramuscular Use - Lyophilized - Single-Dose Vial
-
PRINCIPAL DISPLAY PANEL – Leucovorin Calcium for Injection, USP 100 mg CartonNDC 71288-161-20 - Rx Only - 1 Single-Dose Vial - Leucovorin Calcium for Injection, USP - 100 mg per vial - For Intravenous or Intramuscular Use - Lyophilized
-
PRINCIPAL DISPLAY PANEL – Leucovorin Calcium for Injection, USP 200 mg Vial LabelNDC 71288-162-30 - Rx Only - Leucovorin Calcium for Injection, USP - 200 mg per vial - For Intravenous or Intramuscular Use - Lyophilized - Single-Dose Vial
-
PRINCIPAL DISPLAY PANEL – Leucovorin Calcium for Injection, USP 200 mg CartonNDC 71288-162-30 - Rx Only - 1 Single-Dose Vial - Leucovorin Calcium for Injection, USP - 200 mg per vial - For Intravenous or Intramuscular Use - Lyophilized
-
PRINCIPAL DISPLAY PANEL – Leucovorin Calcium for Injection, USP 350 mg Vial LabelNDC 71288-163-30 - Rx Only - Leucovorin Calcium for Injection, USP - 350 mg per vial - For Intravenous or Intramuscular Use - Lyophilized - Single-Dose Vial
-
PRINCIPAL DISPLAY PANEL – Leucovorin Calcium for Injection, USP 350 mg CartonNDC 71288-163-30 - Rx Only - 1 Single-Dose Vial - Leucovorin Calcium for Injection, USP - 350 mg per vial - For Intravenous or Intramuscular Use - Lyophilized
-
PRINCIPAL DISPLAY PANEL – Leucovorin Calcium for Injection, USP 500 mg Vial LabelNDC 71288-164-50 - Rx Only - Leucovorin Calcium for Injection, USP - 500 mg per vial - For Intravenous or Intramuscular Use - Lyophilized - Single-Dose Vial
-
PRINCIPAL DISPLAY PANEL – Leucovorin Calcium for Injection, USP 500 mg CartonNDC 71288-164-50 - Rx Only - 1 Single-Dose Vial - Leucovorin Calcium for Injection, USP - 500 mg per vial - For Intravenous or Intramuscular Use - Lyophilized
-
INGREDIENTS AND APPEARANCEProduct Information