Label: TRANEXAMIC ACID injection
- NDC Code(s): 70518-4089-0, 70518-4089-1
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 43066-008
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use - TRANEXAMIC ACID INJECTIONsafely and effectively. See full prescribing information for - TRANEXAMIC ACID INJECTION. TRANEXAMIC ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETranexamic acid injection is indicated in patients with hemophilia for short-term use (2 to 8 days) to reduce or prevent hemorrhage and reduce the need for replacement therapy during and following ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dose of Tranexamic acid is 10 mg/kg actual body weight intravenously administered as a single dose, immediately before tooth extractions. Infuse no more ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: 1000 mg tranexamic acid (100 mg/mL) clear and colorless solution in 10 mL single-dose vials
-
4 CONTRAINDICATIONSTranexamic acid Injection is contraindicated: In patients with subarachnoid hemorrhage. Anecdotal experience indicates that cerebral edema and cerebral infarction may be caused by Tranexamic acid ...
-
5 WARNINGS AND PRECAUTIONS5.1 Thromboembolic Risk - Tranexamic acid is contraindicated in patients with active intravascular clotting. Tranexamic acid is an antifibrinolytic and may increase the risk of thromboembolic ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Thromboembolic Risk - [see - Warnings and Precautions (5.1)] Seizures - [see ...
-
7 DRUG INTERACTIONS7.1 Prothrombotic Medical Products - Avoid concomitant use of Tranexamic acid with medical products that are prothrombotic because concomitant use can further increase the risk of thromboembolic ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published studies, case series and case reports with tranexamic acid use in pregnant women in the second and third trimester and at the time of ...
-
10 OVERDOSAGECases of overdosage of Tranexamic acid have been reported. Based on these reports, symptoms of overdosage may be gastrointestinal, e.g., nausea, vomiting, diarrhea; hypotensive, e.g., orthostatic ...
-
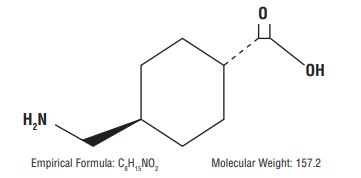
11 DESCRIPTIONTranexamic acid is trans-4-(aminomethyl)cyclohexanecarboxylic acid, an antifibrinolytic agent. Tranexamic acid is a white crystalline powder. The structural formula is - Each mL of the sterile ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tranexamic acid is a synthetic lysine amino acid derivative, which diminishes the dissolution of hemostatic fibrin by plasmin. In the presence of tranexamic acid, the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Tranexamic acid was not carcinogenic in a 2-year study in rats and mice at oral doses up to 3 and 5.3 g/kg/day, which are approximately ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTranexamic Acid Injection USP, 100 mg/mL is supplied as a sterile, clear, colorless, preservative-free aqueous solution in single use 10 mL vials, packed in boxes of 10. NDC: 70518-4089-00 - NDC ...
-
17 PATIENT COUNSELING INFORMATIONThromboembolic Risk - Inform patients that Tranexamic acid may increase the risk of venous and arterial thrombosis or thromboembolism and to contact their healthcare provider for any signs or ...
-
SPL UNCLASSIFIED SECTIONRepackaged and Distributed By: Remedy Repack, Inc. 625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762
-
PRINCIPAL DISPLAY PANELDRUG: TRANEXAMIC ACID - GENERIC: tranexamic acid - DOSAGE: INJECTION - ADMINSTRATION: INTRAVENOUS - NDC: 70518-4089-0 - NDC: 70518-4089-1 - PACKAGING: 1 in 1 CARTON - OUTER PACKAGING: 10 in 1 BOX - PACKAGING; 10 ...
-
INGREDIENTS AND APPEARANCEProduct Information