Label: DILTIAZEM HYDROCHLORIDE injection, solution
- NDC Code(s): 25021-319-05, 25021-319-10, 25021-319-25
- Packager: Sagent Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONSAGENT® Rx only
-
DESCRIPTION
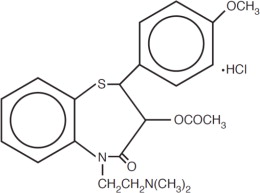
Diltiazem Hydrochloride Injection is a calcium ion influx inhibitor (slow channel blocker or calcium channel antagonist). Chemically, diltiazem hydrochloride is ...
-
CLINICAL PHARMACOLOGY
Mechanisms of Action - Diltiazem inhibits the influx of calcium (Ca2+) ions during membrane depolarization of cardiac and vascular smooth muscle. The therapeutic benefits of diltiazem in ...
-
INDICATIONS AND USAGE
Diltiazem hydrochloride injection is indicated for the following: Atrial Fibrillation or Atrial Flutter - Temporary control of rapid ventricular rate in atrial fibrillation or atrial flutter ...
-
CONTRAINDICATIONS
Diltiazem hydrochloride injection is contraindicated in: Patients with sick sinus syndrome except in the presence of a functioning ventricular pacemaker. Patients with second- or third-degree ...
-
WARNINGS
Cardiac Conduction - Diltiazem prolongs AV nodal conduction and refractoriness that may rarely result in second- or third-degree AV block in sinus rhythm. Concomitant use of diltiazem with ...
-
PRECAUTIONS
General - Diltiazem hydrochloride is extensively metabolized by the liver and excreted by the kidneys and in bile. The drug should be used with caution in patients with impaired renal or hepatic ...
-
ADVERSE REACTIONS
The following adverse reaction rates are based on the use of diltiazem hydrochloride injection in over 400 domestic clinical trial patients with atrial fibrillation/flutter or PSVT under ...
-
OVERDOSAGE
Overdosage experience is limited. In the event of overdosage or an exaggerated response, appropriate supportive measures should be employed. The following measures may be considered: Bradycardia ...
-
DOSAGE AND ADMINISTRATION
Direct Intravenous Single Injections (Bolus) The initial dose of diltiazem hydrochloride injection should be 0.25 mg/kg actual body weight as a bolus administered over 2 minutes (20 mg is a ...
-
HOW SUPPLIED
Diltiazem Hydrochloride Injection is supplied as follows: NDCDiltiazem Hydrochloride Injection (5 mg per mL)Package Factor - 25021-319-05 - 25 mg per 5 mL Single-Dose Vial - 10 vials per ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Vial Label - NDC 25021-319-05 - Rx only - Diltiazem HCl Injection - 25 mg per 5 mL - (5 mg per mL) For Direct Intravenous Bolus - Injection and ...
-
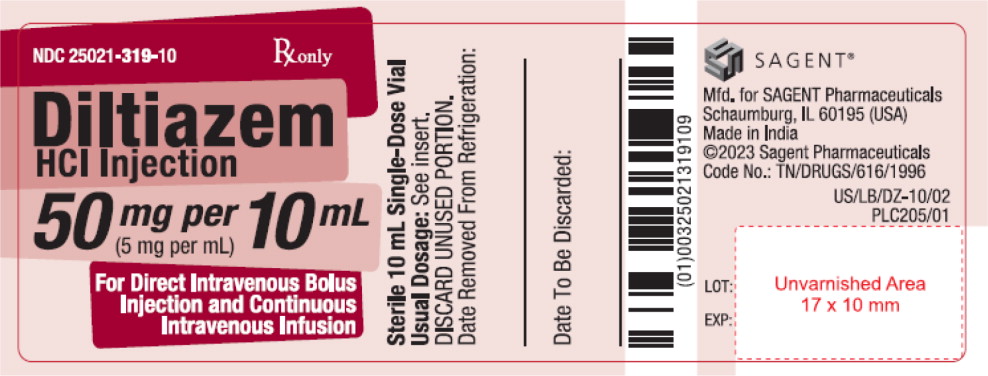
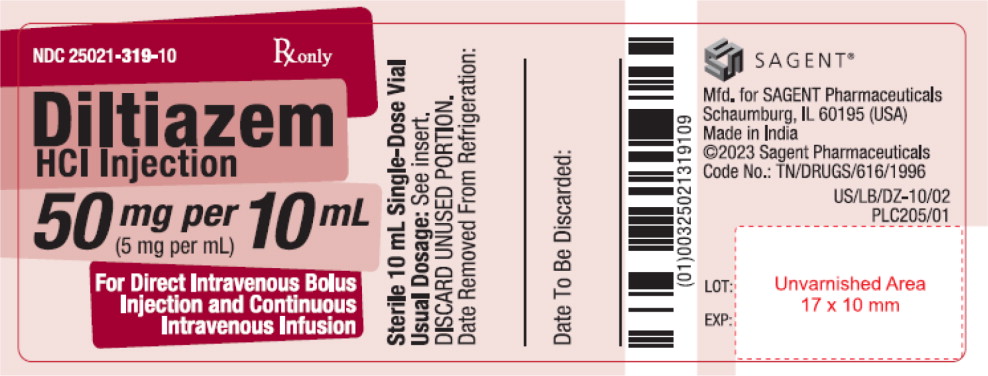
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Vial Label - NDC 25021-319-10 - Rx only - Diltiazem HCl Injection - 50 mg per 10 mL - (5 mg per mL) For Direct Intravenous Bolus - Injection and ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Vial Label - NDC 25021-319-25 - Rx only - Diltiazem HCl Injection - 125 mg per 25 mL - (5 mg per mL) For Direct Intravenous Bolus - Injection and ...
-
INGREDIENTS AND APPEARANCEProduct Information