Label: DAPSONE tablet
- NDC Code(s): 82804-988-00, 82804-988-11, 82804-988-30, 82804-988-55, view more
- Packager: Proficient Rx LP
- This is a repackaged label.
- Source NDC Code(s): 69367-378, 69367-379
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
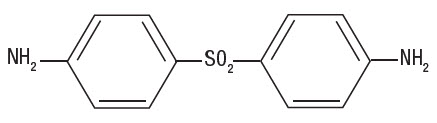
DESCRIPTIONDapsone-USP, 4,4'-diaminodiphenylsulfone (DDS), is a primary treatment for Dermatitis herpetiformis. It is an antibacterial drug for susceptible cases of leprosy. It is a white, odorless ...
-
CLINICAL PHARMACOLOGYActions - The mechanism of action in Dermatitis herpetiformis has not been established. By the kinetic method in mice, Dapsone is bactericidal as well as bacteriostatic against Mycobacterium ...
-
INDICATIONS AND USAGEDermatitis herpetiformis: (D.H.) Leprosy: All forms of leprosy except for cases of proven Dapsone resistance.
-
CONTRAINDICATIONSHypersensitivity to Dapsone and/or its derivatives.
-
WARNINGSThe patient should be warned to respond to the presence of clinical signs such as sore throat, fever, pallor, purpura or jaundice. Deaths associated with the administration of Dapsone have been ...
-
PRECAUTIONSGeneral - Hemolysis and Heinz body formation may be exaggerated in individuals with a glucose-6-phosphate dehydrogenase (G6PD) deficiency, or methemoglobin reductase deficiency, or hemoglobin M ...
-
ADVERSE REACTIONSIn addition to the warnings listed above, the following syndromes and serious reactions have been reported in patients on Dapsone. Hematologic Effects - Dose-related hemolysis is the most common ...
-
OVERDOSAGENausea, vomiting, hyperexcitability can appear a few minutes up to 24 hours after ingestion of an overdosage. Methemoglobin induced depression, convulsions or severe cyanosis requires prompt ...
-
DOSAGE AND ADMINISTRATIONDermatitis herpetiformis - The dosage should be individually titrated starting in adults with 50 mg daily and correspondingly smaller doses in children. If full control is not achieved within the ...
-
HOW SUPPLIEDDapsone Tablets USP, 25 mg are available as round white scored tablets, debossed "25" above and "102" below the score and on the obverse "JACOBUS" in light and child-resistant bottles. Bottle of ...
-
REFERENCES1. Lee, B., et al., Zidovudine, Trimethoprim, and Dapsone Pharmacokinetic Interactions in Patients with HIV Infection. Antimicrobial Agents and Chemotherapy, May 1996; 1231-1236. 2. Lee, B., et ...
-
STORAGE AND HANDLINGStore at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from light. Rx only. Keep this and all drugs out of the reach of ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Everest Life Sciences LLC - Plainsboro, NJ 08536 - Distributed by: Westminster Pharmaceuticals, LLC - Nashville, TN 37217 - Repackaged and Relabeled by: Proficient Rx LP - Thousand ...
-
PRINCIPAL DISPLAY PANEL - 25 mg Tablet Bottle LabelNDC 82804-988-30 - DAPSONE - Tablets, USP - 25 mg - Caution: Federal law prohibits dispensing without - prescription. Dispense this product in a well closed, light-resistant container with child ...
-
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle LabelNDC 82804-989-30 - DAPSONE - Tablets, USP - 100 mg - Caution: Federal law prohibits dispensing without - prescription. Dispense this product in a well closed, light-resistant container with child ...
-
INGREDIENTS AND APPEARANCEProduct Information