Label: TRETINOIN capsule
- NDC Code(s): 10370-268-01
- Packager: Endo USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 1, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TRETINOIN CAPSULES safely and effectively. See full prescribing information for TRETINOIN CAPSULES. TRETINOIN capsules, for oral ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: EMBRYO-FETAL TOXICITY and DIFFERENTIATION SYNDROME
- Tretinoin capsules can cause embryo-fetal loss and malformations when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Females of reproductive potential must have a negative pregnancy test before initiating tretinoin capsules. Advise females of reproductive potential to use two effective methods of contraception during treatment with tretinoin capsules and for 1 month after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with tretinoin capsules and for 1 week after the last dose [see Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.3)].
- Differentiation Syndrome, which can be life-threatening or fatal, occurred in about 26% of patients with APL who received tretinoin capsules. At first signs or symptoms of this syndrome, immediately initiate high-dose corticosteroid therapy and hemodynamic monitoring until resolution of signs and symptoms. Consider withholding tretinoin capsules for moderate and severe Differentiation Syndrome until resolution [see Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGETretinoin capsules are indicated for the induction of remission in adults and pediatric patients 1 year of age and older with acute promyelocytic leukemia (APL) characterized by the presence of ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Safety Information - Verify pregnancy status in females of reproductive potential prior to initiating tretinoin capsules. Females of reproductive potential must have a negative ...
-
3 DOSAGE FORMS AND STRENGTHSCapsules: 10 mg, two-tone (lengthwise) with reddish-brown opaque and yellow gelatin shell, imprinted with “TR” with black ink on the yellow side.
-
4 CONTRAINDICATIONSTretinoin capsules are contraindicated in patients with a known hypersensitivity to tretinoin capsules, any of its components, or other retinoids. Reactions have included rash, pruritus, face ...
-
5 WARNINGS AND PRECAUTIONS5.1 Embryo-Fetal Toxicity - Tretinoin capsules can cause embryo-fetal loss and malformations when administered to a pregnant woman. Tretinoin capsules are a retinoid and there is an increased ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Differentiation Syndrome [see Warnings and Precautions (5.2)] Leukocytosis [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Effects of Other Drugs on Tretinoin Capsules - Strong or moderate CYP3A Inhibitors - Avoid concomitant use of tretinoin capsules with strong CYP3A inhibitors if possible and monitor more ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings in animals and its mechanism of action [see Clinical Pharmacology (12.1)], tretinoin capsules can cause embryo-fetal loss and malformations when ...
-
10 OVERDOSAGEIn case of overdose with tretinoin capsules, reversible signs of hypervitaminosis A (headache, nausea, vomiting, mucocutaneous symptoms) can appear. Overdosage with other retinoids has been ...
-
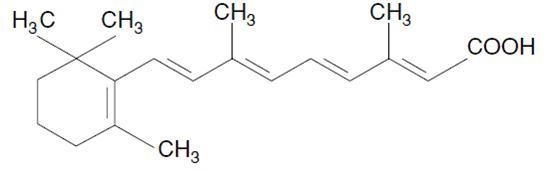
11 DESCRIPTIONTretinoin is a retinoid. The chemical name is all-trans retinoic acid. The molecular formula is C20H28O2 and the molecular weight is 300.44 g/mol. The structural formula is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tretinoin induces cytodifferentiation and decreased proliferation of APL cells in culture and in vivo. In APL patients, tretinoin treatment produces an initial ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No 2-year carcinogenicity studies in rodents have been conducted with tretinoin. In a carcinogenicity study, female B5D2F1 mice ...
-
14 CLINICAL STUDIESThe efficacy of tretinoin capsules has been evaluated in 114 previously treated patients and in 67 previously untreated (“de novo”) patients with APL in one open-label, uncontrolled single ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTretinoin capsules are supplied as 10 mg capsules, two-tone (lengthwise) with reddish-brown opaque and yellow gelatin shell, imprinted with “TR” with black ink on the yellow side. Supplied in ...
-
17 PATIENT COUNSELING INFORMATIONEmbryo-Fetal Toxicity - Advise female patients of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANELNDC 10370-268-01 - Tretinoin Capsules 10 mg - CAUSES BIRTH DEFECTS - DO NOT GET PREGNANT - Contains the active ingredient found in Vesanoid* PHARMACIST: PLEASE DISPENSE WITH ATTACHED PATIENT ...
-
INGREDIENTS AND APPEARANCEProduct Information