Label: ZAVZPRET- zavegepant spray
- NDC Code(s): 0069-3500-01, 0069-3500-02
- Packager: Pfizer Laboratories Div Pfizer Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 31, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZAVZPRET safely and effectively. See full prescribing information for ZAVZPRET. ZAVZPRET™ (zavegepant) nasal spray - Initial U.S ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZAVZPRET is indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use - ZAVZPRET is not indicated for the preventive treatment of migraine.
-
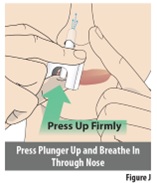
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The recommended dose of ZAVZPRET is 10 mg given as a single spray in one nostril, as needed. The maximum dose that may be given in a 24-hour period is 10 mg (one spray) ...
-
3 DOSAGE FORMS AND STRENGTHSNasal spray: 10 mg of zavegepant per device. Each unit-dose nasal spray device delivers a single spray containing 10 mg of zavegepant.
-
4 CONTRAINDICATIONSZAVZPRET is contraindicated in patients with a history of hypersensitivity reaction to zavegepant or any of the components of ZAVZPRET [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Hypersensitivity reactions, including facial swelling and urticaria, have occurred in patients treated with ZAVZPRET in clinical studies. If a hypersensitivity ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling: • Hypersensitivity Reactions [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 OATP1B3 or NTCP Inhibitors - Concomitant administration of ZAVZPRET with inhibitors of the organic anion transporting polypeptide 1B3 (OATP1B3) or sodium taurocholate co-transporting ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with the use of ZAVZPRET in pregnant women. No adverse developmental effects were observed following ...
-
11 DESCRIPTION ZAVZPRET (zavegepant) nasal spray contains zavegepant hydrochloride, a calcitonin gene-related peptide receptor antagonist. Zavegepant hydrochloride is described chemically as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Zavegepant is a calcitonin gene-related peptide (CGRP) receptor antagonist. 12.2 Pharmacodynamics - The relationship between pharmacodynamic activity and the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Intranasal administration of zavegepant (0, 0.3, 0.8, or 2.5 mg/day) to Tg.rasH2 mice for 26 weeks resulted in no ...
-
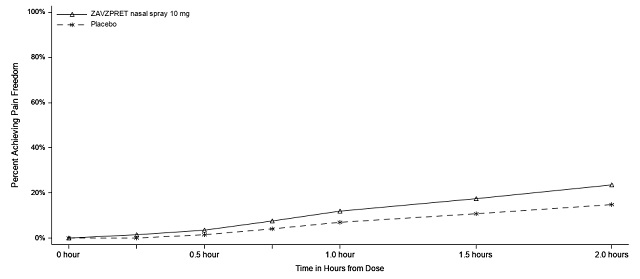
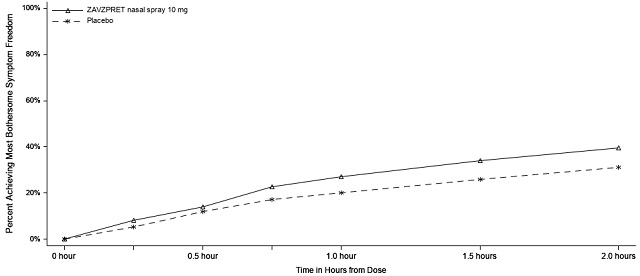
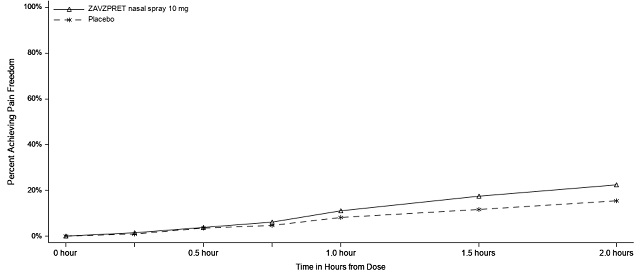
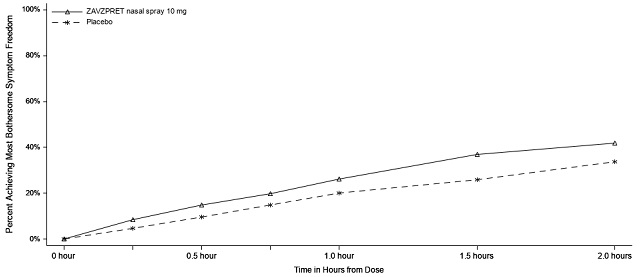
14 CLINICAL STUDIES The efficacy of ZAVZPRET for the acute treatment of migraine with or without aura in adults was demonstrated in two randomized, double-blind, placebo-controlled trials (Study 1 and Study 2). In ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - ZAVZPRET nasal spray (NDC 0069-3500-01) contains 10 mg zavegepant and is supplied as a ready-to-use, unit-dose disposable device. Each carton contains 6 units (NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Hypersensitivity Reactions - Inform patients about the signs and symptoms of ...
-
Patient Package Insert PATIENT INFORMATION - ZAVZPRET™ (zav-spret) (zavegepant) nasal spray - What is ZAVZPRET? ZAVZPRET is a prescription medicine used in adults for the acute treatment of migraine attacks ...
-
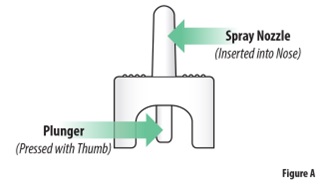
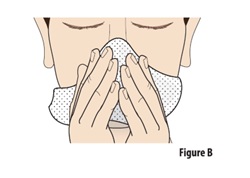
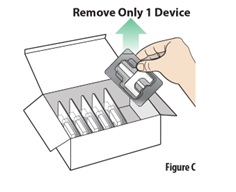
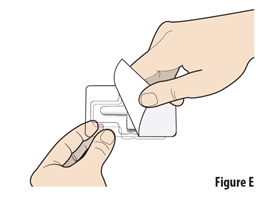
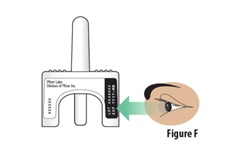
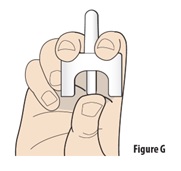
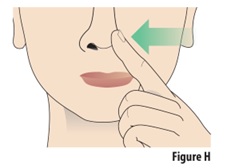
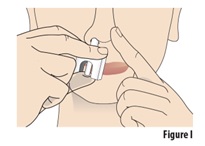
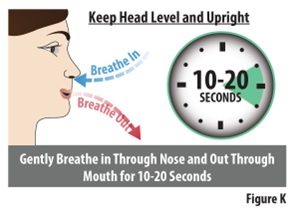
Instructions for Use INSTRUCTIONS FOR USE - ZAVZPRET [zav-spret] (zavegepant) nasal spray - For Nasal Use Only - This Instructions for Use contains information on how to give a single dose (10 mg) with ...
-
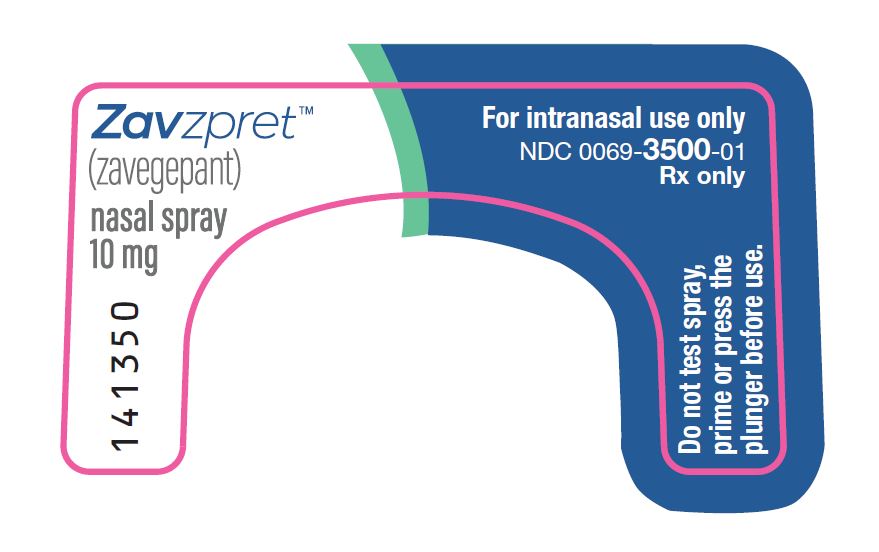
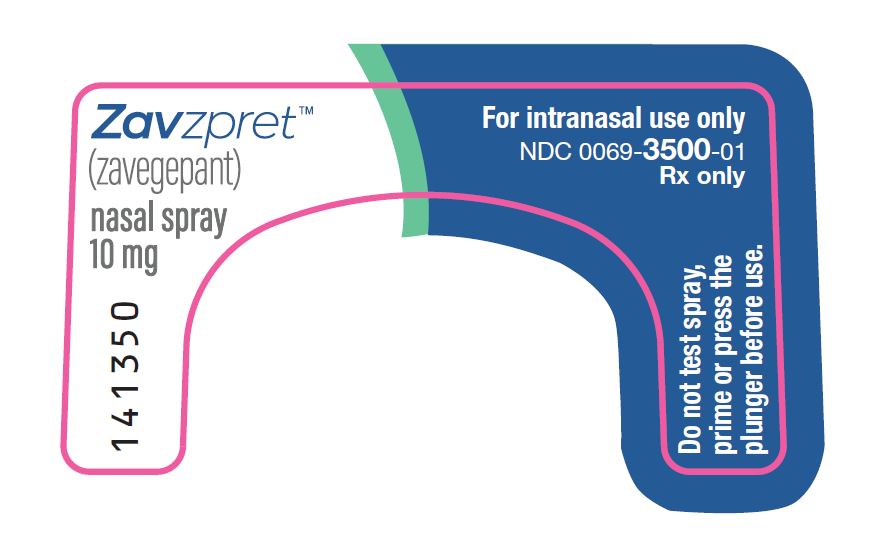
PRINCIPAL DISPLAY PANEL – Nasal Spray Device Label Front ZavzpretTM - (zavegepant) nasal spray - 10 mg - For intranasal use only - NDC 0069-3500-01 - Rx only - Do not test spray, prime or press the - plunger before use.
-
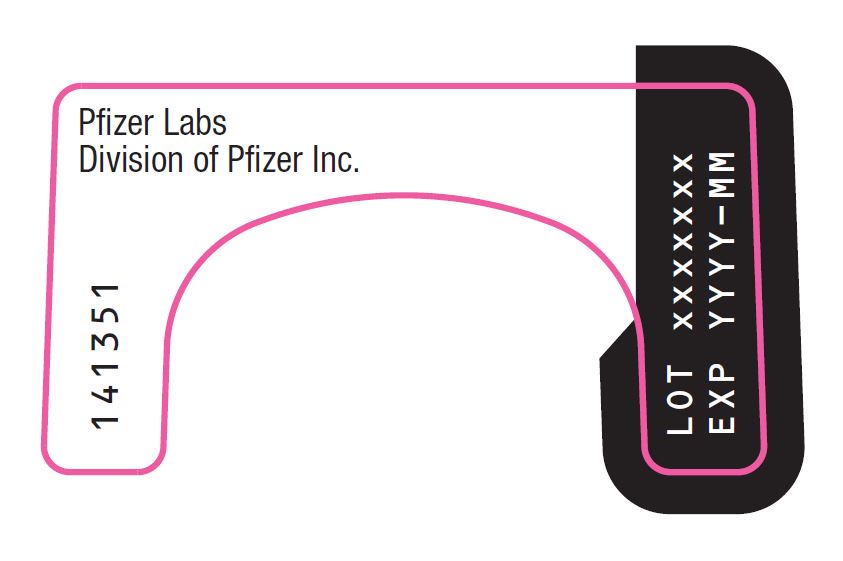
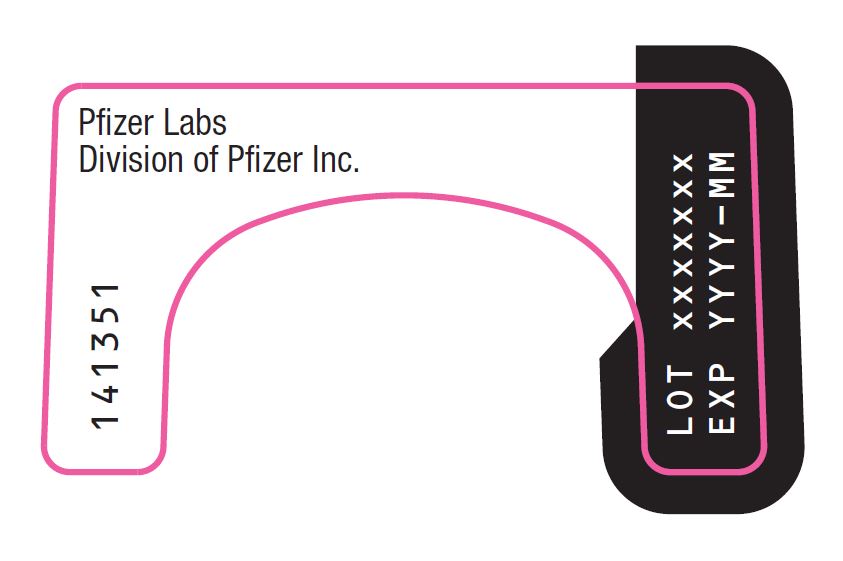
PRINCIPAL DISPLAY PANEL – Nasal Spray Device Label Back Pfizer Labs - Division of Pfizer Inc. LOT xxxxxxx - EXP YYYY-MM
-
PRINCIPAL DISPLAY PANEL – Nasal Spray Blister Foil Label NDC 0069-3500-01 - Rx only - PEEL OFF - ZavzpretTM - (zavegepant) nasal spray 10 mg - Each unit-dose device contains 10 mg of zavegepant. 1 spray (10 mg dose) per unit. Do not test spray, prime or press ...
-
PRINCIPAL DISPLAY PANEL – Nasal Spray Carton NDC 0069-3500-02 - Contains 6 of NDC 0069-3500-01 - Rx only - ZavzpretTM - (zavegepant) nasal spray 10 mg - This box contains: • 6 unit-dose nasal spray devices. Each unit-dose nasal spray device - contains ...
-
INGREDIENTS AND APPEARANCEProduct Information