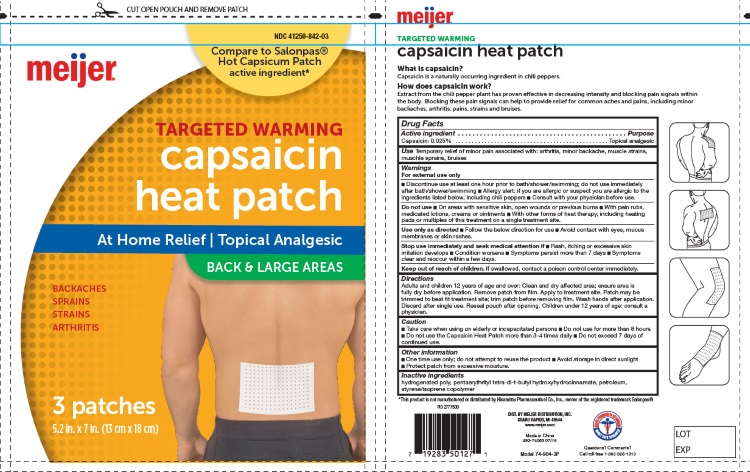
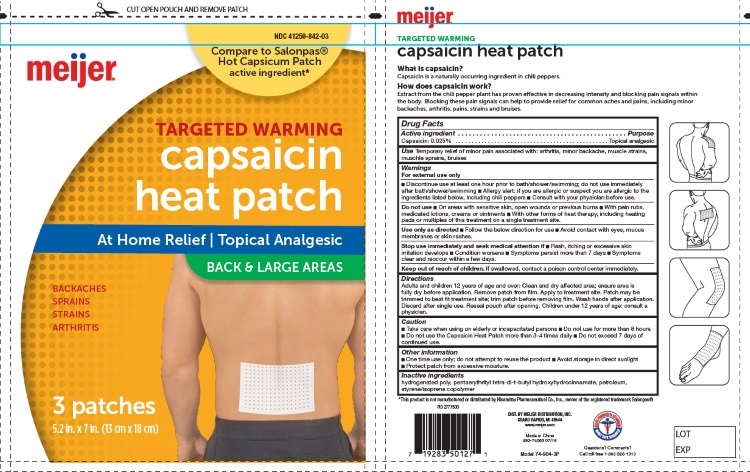
Label: CAPSAICIN HEAT PATCHES BACK AND LARGE AREAS- capsaicin patch
- NDC Code(s): 41250-842-03
- Packager: Meijer Distribution Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- For External Use Only
- Uses
- Purpose
- Active Ingredients
- Inactive Ingredients:
-
Directions for Use:
For Use on Adults and Childrens 12 Years of Age or Older
- Clean and dru affected area; ensure area is fully dry before application
- Remove patch from film
- Apply to treatement site
- Patch may be trimmerd to best fit treatment site; trim patch before removing film
- Wash hands after application
- Discard after signle use
Caution:
- Take care when using on elderly ot incapacited persons
- Do not use more than 8 hours
- Do not use the Capsaicin Heat Patch more than 3-4 times daily
- Do not exceed 7 days of continue use
- Keep Out of Reach of Children
- Storage and Care:
- Do Not Use:
- Use Only as Directed:
- Stop Use immediately and Seek Medical Attention if:
- QUESTIONS
- LABEL
-
INGREDIENTS AND APPEARANCE
CAPSAICIN HEAT PATCHES BACK AND LARGE AREAS
capsaicin patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41250-842 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.025 g in 100 g Inactive Ingredients Ingredient Name Strength PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) HYDROGENATED POLYDECENE (550 MW) (UNII: U333RI6EB7) LIQUID PETROLEUM (UNII: 6ZAE7X688J) STYRENE/ACRYLAMIDE COPOLYMER (MW 500000) (UNII: 5Z4DPO246A) Product Characteristics Color Score Shape RECTANGLE Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41250-842-03 3 in 1 BOX 07/01/2018 1 9 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/01/2017 Labeler - Meijer Distribution Inc (006959555) Establishment Name Address ID/FEI Business Operations Foshan Aqua Gel Biotech Co.,Ltd. 529128763 manufacture(41250-842)