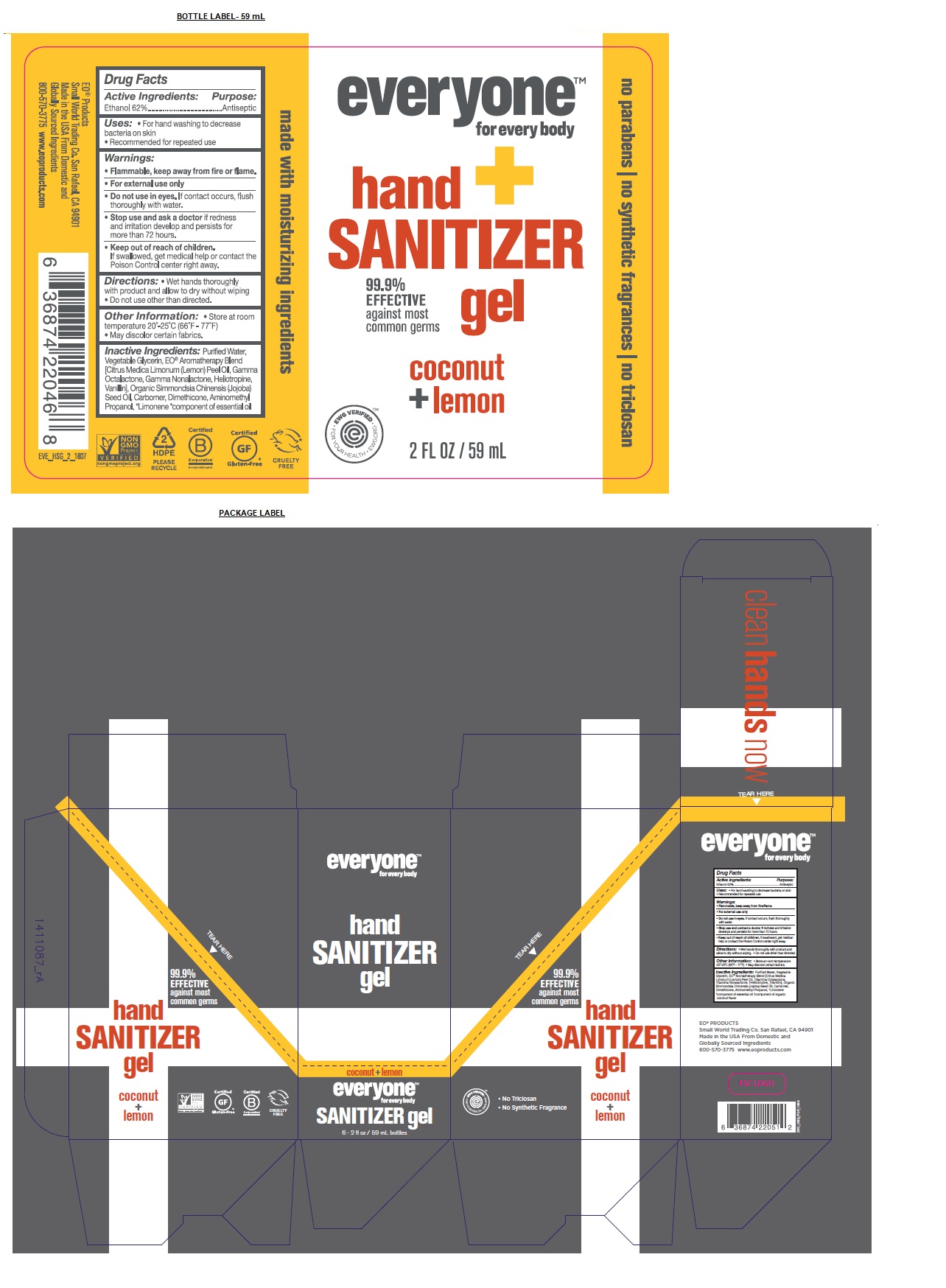
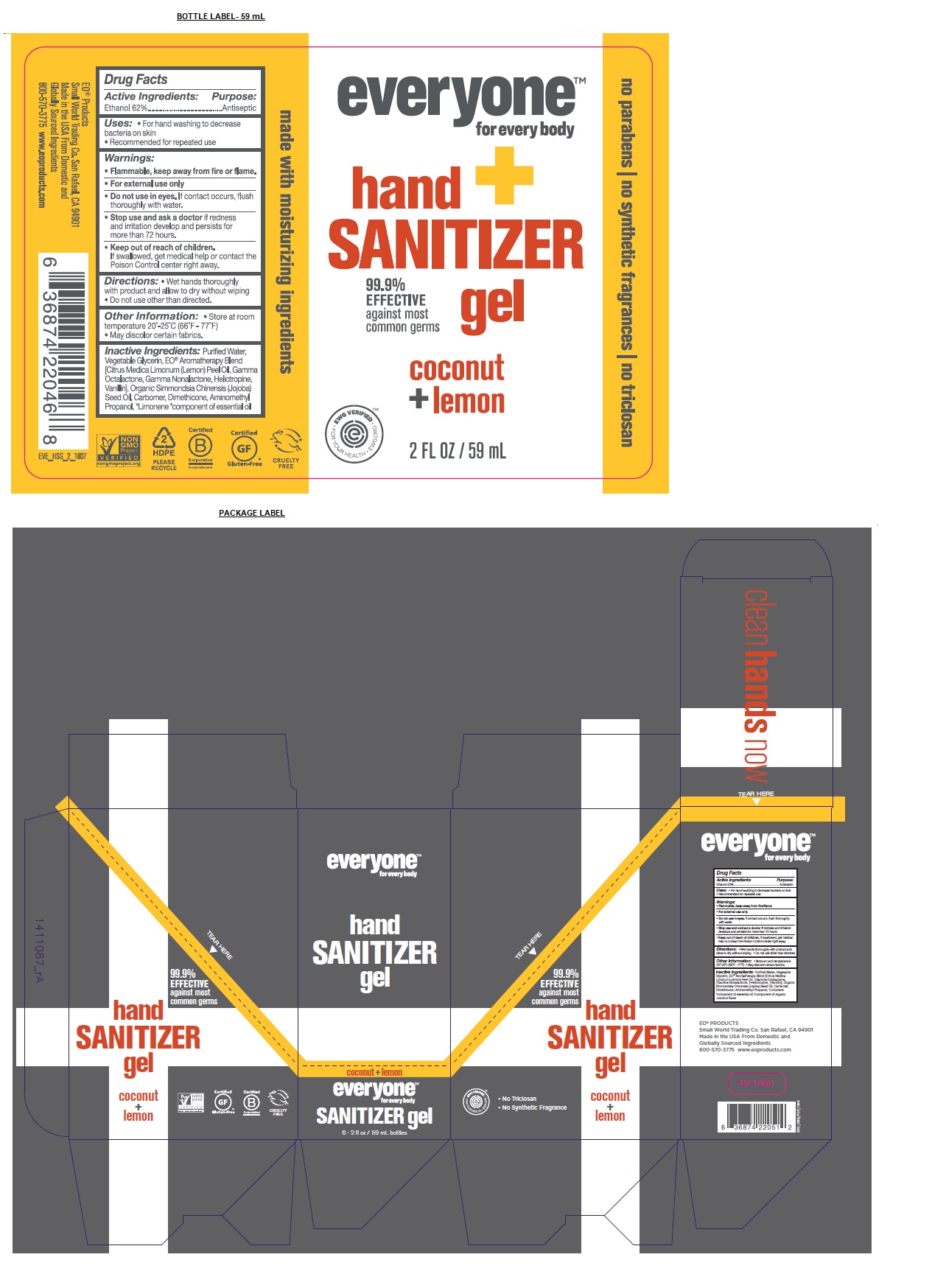
Label: EVERYONE HAND SANITIZER COCONUT LEMON- alcohol gel
- NDC Code(s): 54748-401-02, 54748-401-05, 54748-401-08, 54748-401-09
- Packager: EO Products, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients:
- Purpose:
- Uses:
- Warnings:
- KEEP OUT OF REACH OF CHILDREN
- Directions:
-
Inactive Ingredients:
Purified Water, Vegetable Glycerin, EO® Aromatherapy Blend [Citrus Medica Limonum (Lemon) Peel Oil, Ethanol, Gamma Octalactone, Gamma Nonalactone, Heliotropine, Vanillin], Organic Simmondsia Chinensis (Jojoba) Seed Oil, Carbomer, Dimethicone, Aminomethyl Propanol, *Limonene
*component of essential oil
- Other Information:
-
SPL UNCLASSIFIED SECTION
99.9% EFFECTIVE against most common germs
- No Disodium EDTA
clean hands now
no parabens / no synthetic fragrances / no triclosan
made with moisturizing ingredients
EO® PRODUCTS
Small World Trading Co. San Rafael, CA 94901
Made in the USA From Domestic and Globally Sourced Ingredients
800-570-3775 www.eoproducts.com
- Packaging
-
INGREDIENTS AND APPEARANCE
EVERYONE HAND SANITIZER COCONUT LEMON
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54748-401 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) LEMON OIL (UNII: I9GRO824LL) .GAMMA.-OCTALACTONE (UNII: UHD6M52X0K) .GAMMA.-NONALACTONE (UNII: I1XGH66S8P) PIPERONAL (UNII: KE109YAK00) VANILLIN (UNII: CHI530446X) JOJOBA OIL (UNII: 724GKU717M) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) DIMETHICONE, UNSPECIFIED (UNII: 92RU3N3Y1O) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) LIMONENE, (+)- (UNII: GFD7C86Q1W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54748-401-02 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/07/2013 02/15/2017 2 NDC:54748-401-05 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/31/2018 3 NDC:54748-401-09 6 in 1 PACKAGE 08/31/2018 3 NDC:54748-401-05 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:54748-401-08 1 in 1 CASE 05/15/2015 4 237 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 06/07/2013 Labeler - EO Products, LLC (786611210) Establishment Name Address ID/FEI Business Operations EO Products, LLC 786611210 manufacture(54748-401)