Label: ALLOPURINOL SODIUM injection, powder, lyophilized, for solution
- NDC Code(s): 0143-9533-01
- Packager: Hikma Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Allopurinol Sodium for Injection safely and effectively. See full prescribing information for Allopurinol Sodium for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAllopurinol Sodium for Injection is indicated for the management of adult and pediatric patients with leukemia, lymphoma, and solid tumor malignancies who are receiving cancer therapy which causes ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Initiate therapy with Allopurinol Sodium for Injection 24 to 48 hours before the start of chemotherapy known to cause tumor cell lysis. Additionally, administer fluids ...
-
3 DOSAGE FORMS AND STRENGTHSFor Injection: 500 mg of allopurinol as a sterile, white lyophilized powder or cake in a single-dose vial for reconstitution.
-
4 CONTRAINDICATIONSAllopurinol Sodium for Injection is contraindicated in patients with a history of severe reaction to any formulation of allopurinol.
-
5 WARNINGS AND PRECAUTIONS5.1 Skin Rash and Hypersensitivity - Serious and sometimes fatal dermatologic reactions, including toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), and drug reaction with ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Skin Rash and Hypersensitivity [see Warnings and Precautions (5.1)] Renal Function Impairment [see ...
-
7 DRUG INTERACTIONSClinically important interactions with the drugs listed below were observed in patients undergoing treatment with an oral allopurinol formulation. 7.1 Drugs Known to Affect the Occurrence of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings in animals, Allopurinol Sodium for Injection may cause fetal harm when administered to a pregnant woman. Adverse developmental outcomes have ...
-
10 OVERDOSAGEIn the management of overdosage, there is no specific antidote for Allopurinol Sodium for Injection. Both allopurinol and oxypurinol are dialyzable; however, the usefulness of hemodialysis or ...
-
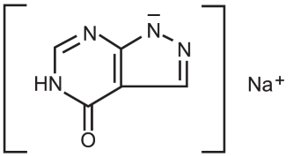
11 DESCRIPTIONAllopurinol Sodium for Injection, a xanthine oxidase inhibitor, is a sterile, white, lyophilized powder or cake, in a single-dose vial for reconstitution. Each vial contains 500 mg of allopurinol ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Allopurinol is a structural analogue of the natural purine base, hypoxanthine. Allopurinol and its oxypurinol metabolite inhibitor xanthine oxidase, the enzyme ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Allopurinol was administered at doses up to 20 mg/kg/day to mice and rats for the majority of their life span. No evidence of ...
-
14 CLINICAL STUDIESA compassionate use trial of Allopurinol Sodium for Injection conducted in the United States from 1977 through 1989 included 718 evaluable patients with malignancies requiring treatment with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSTERILE SINGLE DOSE VIAL FOR INTRAVENOUS INFUSION. Allopurinol Sodium for Injection, 50 mL flint glass vials with rubber stoppers each containing allopurinol sodium equivalent to 500 mg of ...
-
17 PATIENT COUNSELING INFORMATIONSkin Rash and Hypersensitivity - Inform patients that Allopurinol Sodium for Injection may increase the risk of serious and sometimes fatal dermatologic reactions, including toxic epidermal ...
-
PRINCIPAL DISPLAY PANEL – 500 mg/vialNDC 0143-9533-01 Rx only - Allopurinol Sodium - for Injection - 500 mg per vial - For Intravenous Infusion - Sterile Single Use Vial - NDC 0143-9533-01 Rx only - Allopurinol Sodium - for ...
-
INGREDIENTS AND APPEARANCEProduct Information