Label: OTREXUP- methotrexate injection, solution
-
NDC Code(s):
54436-010-01,
54436-010-02,
54436-010-03,
54436-010-04, view more54436-012-01, 54436-012-02, 54436-012-03, 54436-012-04, 54436-015-01, 54436-015-02, 54436-015-03, 54436-015-04, 54436-017-01, 54436-017-02, 54436-017-03, 54436-017-04, 54436-020-01, 54436-020-02, 54436-020-03, 54436-020-04, 54436-022-01, 54436-022-02, 54436-022-03, 54436-022-04, 54436-025-01, 54436-025-02, 54436-025-03, 54436-025-04, 54436-075-01, 54436-075-02, 54436-075-04
- Packager: Antares Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OTREXUP™ safely and effectively. See full prescribing information for OTREXUP.
OTREXUP (methotrexate) injection, for subcutaneous use
Initial U.S. Approval: 1953WARNING: SEVERE TOXIC REACTIONS, INCLUDING EMBRYO-FETAL TOXICITY AND DEATH
See full prescribing information for complete boxed warning.
• Serious toxic reactions and death have been reported with the use of methotrexate. Patients should be closely monitored for bone marrow, liver, lung, skin, and kidney toxicities (5.1).
• Methotrexate can cause embryo-fetal toxicity, including fetal death. Use is contraindicated during pregnancy (4). Advise females and males of reproductive potential to use effective contraception during and after treatment with methotrexate (5.2, 8.1, 8.3).
• Unexpectedly severe (sometimes fatal) bone marrow suppression, aplastic anemia, and gastrointestinal toxicity have been reported with concomitant administration of methotrexate (usually in high dosage) along with some nonsteroidal anti-inflammatory drugs (NSAIDs) (5.1).
• Hepatotoxicity, fibrosis, and cirrhosis may occur after prolonged use (5.1).
• Methotrexate may cause interstitial pneumonitis at any time during therapy and has been reported at low doses. Pulmonary symptoms (especially a dry, nonproductive cough) may require interruption of treatment and careful investigation (5.1).
• Diarrhea, ulcerative stomatitis, hemorrhagic enteritis, and death from intestinal perforation may occur (5.1).
• Severe, occasionally fatal, skin reactions have been reported (5.1).
• Potentially fatal opportunistic infections may occur (5.1).
INDICATIONS AND USAGE
Otrexup is a folate analog metabolic inhibitor indicated for the:
• Management of patients with severe, active rheumatoid arthritis (RA) and polyarticular juvenile idiopathic arthritis (pJIA), who are intolerant of or had an inadequate response to first-line therapy (1.1).
• Symptomatic control of severe, recalcitrant, disabling psoriasis in adults who are not adequately responsive to other forms of therapy (1.2).
Limitation of Use
Otrexup is not indicated for the treatment of neoplastic diseases (1.3).
DOSAGE AND ADMINISTRATION
- Otrexup is for once weekly subcutaneous use only. Administer Otrexup in the abdomen or thigh. (2.1).
- Use another formulation of methotrexate for patients requiring oral, intramuscular, intravenous, intra-arterial, or intrathecal dosing, doses less than 10 mg per week, doses above 25 mg per week, high-dose regimens, or dose adjustments of less than 5 mg increments (2.1).
- Starting doses of methotrexate:
- Adjust dose gradually to achieve an optimal response (2.2, 2.3).
DOSAGE FORMS AND STRENGTHS
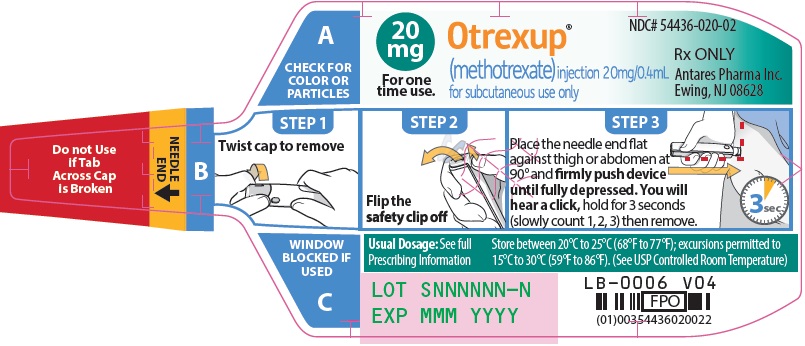
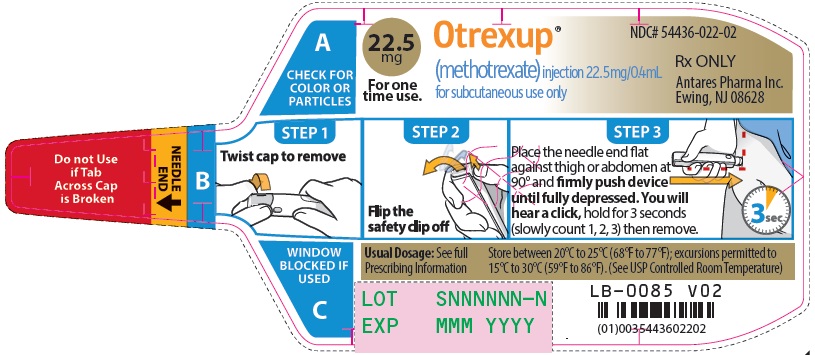
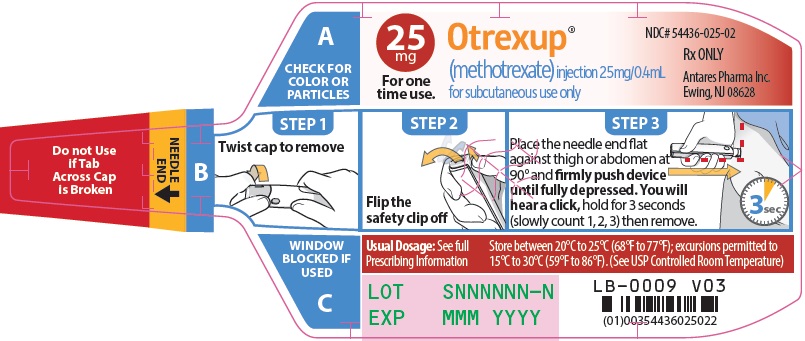
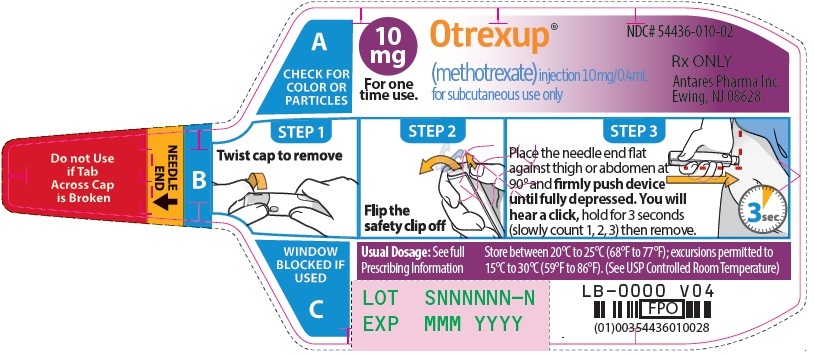
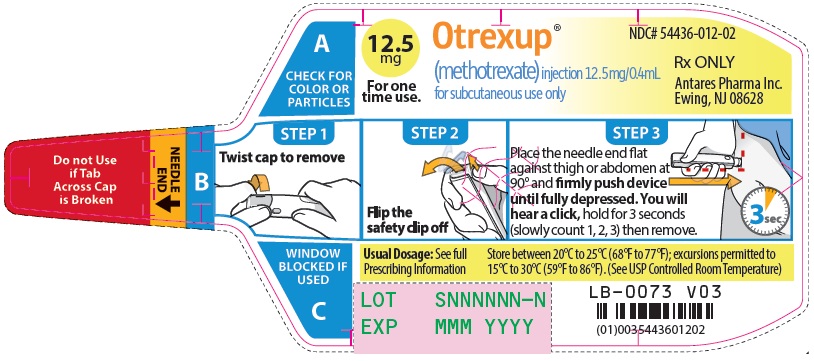
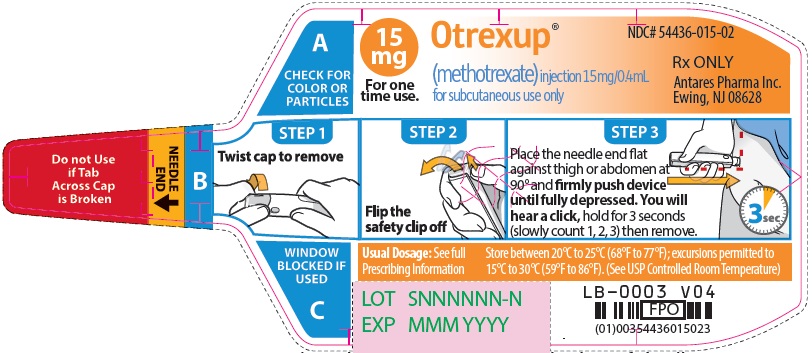
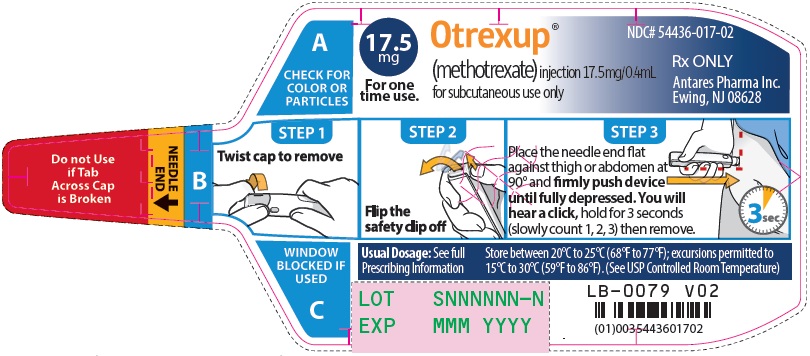
Injection: Single-dose auto-injector delivering 0.4 mL of methotrexate in the following dosage strengths: 10 mg, 12.5 mg, 15 mg, 17.5 mg, 20 mg, 22.5 mg and 25 mg (3).
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Organ system toxicity: Potential for serious toxicity. Only for use by physicians experienced in antimetabolite therapy (5.1).
- Effects on reproduction: May cause impairment of fertility, oligospermia and menstrual dysfunction (5.3, 8.3).
- Laboratory tests: Monitor complete blood counts, renal function and liver function tests (5.4).
- Risks from improper dosing: Mistaken daily use has led to fatal toxicity (5.5).
- Patients with impaired renal function, ascites, or pleural effusions: Elimination is reduced (5.6).
- Dizziness and fatigue: May impair ability to drive or operate machinery (5.7).
ADVERSE REACTIONS
Common adverse reactions are: nausea, abdominal pain, dyspepsia, stomatitis/mouth sores, rash, nasopharyngitis, diarrhea, liver function test abnormalities, vomiting, headache, bronchitis, thrombocytopenia, alopecia, leucopenia, pancytopenia, dizziness, photosensitivity, and “burning of skin lesions” (6).
To report SUSPECTED ADVERSE REACTIONS, contact Antares at 1-855-Otrexup (1-855-687-3987) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Pediatric use: Safety and efficacy of methotrexate, including Otrexup, have not been established in pediatric patients with psoriasis. Safety and efficacy of Otrexup have not been established in pediatric patients with malignancy (8.4).
- Geriatric use: Use caution in dose selection (8.5).
- Lactation: Advise women not to breastfeed (8.2).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SEVERE TOXIC REACTIONS, INCLUDING EMBRYO-FETAL TOXICITY AND DEATH
1 INDICATIONS AND USAGE
1.1 Rheumatoid Arthritis including Polyarticular Juvenile Idiopathic Arthritis
1.2 Psoriasis
1.3 Limitation of Use
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
2.2 Rheumatoid Arthritis including Polyarticular Juvenile Idiopathic Arthritis
2.3 Psoriasis
2.4 Administration and Handling
2.5 Pregnancy testing
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Organ System Toxicity
5.2 Embryo-Fetal Toxicity
5.3 Effects on Reproduction
5.4 Laboratory Tests
5.5 Risks from Improper Dosing
5.6 Patients with Impaired Renal Function, Ascites, or Pleural Effusions
5.7 Dizziness and Fatigue
5.8 Malignant Lymphomas
5.9 Tumor Lysis Syndrome
5.10 Concomitant Radiation Therapy
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Other Adverse Reactions
7 DRUG INTERACTIONS
7.1 Aspirin, Nonsteroidal Anti-Inflammatory Drugs, and Steroids
7.2 Proton Pump Inhibitors (PPIs)
7.3 Oral Antibiotics
7.4 Hepatotoxins
7.5 Theophylline
7.6 Folic Acid and Antifolates
7.7 Mercaptopurine
7.8 Nitrous Oxide
7.9 Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Rheumatoid Arthritis
14.2 Polyarticular Juvenile Idiopathic Arthritis
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SEVERE TOXIC REACTIONS, INCLUDING EMBRYO-FETAL TOXICITY AND DEATH
Otrexup should be used only by physicians whose knowledge and experience include the use of antimetabolite therapy. Because of the possibility of serious toxic reactions (which can be fatal), Otrexup should be used only in patients with psoriasis or rheumatoid arthritis with severe, recalcitrant, disabling disease which is not adequately responsive to other forms of therapy. Deaths have been reported with the use of methotrexate in the treatment of malignancy, psoriasis, and rheumatoid arthritis. Patients should be closely monitored for bone marrow, liver, lung, skin, and kidney toxicities. Patients should be informed by their physician of the risks involved and be under a physician’s care throughout therapy [see Warnings and Precautions (5.1)].
1. Methotrexate can cause embryo-fetal toxicity, including fetal death.
Use is contraindicated during pregnancy. Verify the pregnancy status of females of reproductive potential prior to initiating therapy. Advise females and males of reproductive potential to use effective contraception during and after treatment with Otrexup [see Contraindications (4), Warnings and Precautions (5.2), and Use in Specific Populations (8.1, 8.3)].
2. Methotrexate elimination is reduced in patients with impaired renal functions, ascites, or pleural effusions. Such patients require especially careful monitoring for toxicity, and require dose reduction or, in some cases, discontinuation of Otrexup administration [see Warnings and Precautions (5.6)].
3. Unexpectedly severe (sometimes fatal) bone marrow suppression, aplastic anemia, and gastrointestinal toxicity have been reported with concomitant administration of methotrexate (usually in high dosage) along with some nonsteroidal anti-inflammatory drugs (NSAIDs) [see Warnings and Precautions (5.1) and Drug Interactions (7.1)].
4. Methotrexate causes hepatotoxicity, fibrosis and cirrhosis, but generally only after prolonged use. Acutely, liver enzyme elevations are frequently seen. These are usually transient and asymptomatic, and also do not appear predictive of subsequent hepatic disease. Liver biopsy after sustained use often shows histologic changes, and fibrosis and cirrhosis have been reported; these latter lesions may not be preceded by symptoms or abnormal liver function tests in the psoriasis population. For this reason, periodic liver biopsies are usually recommended for psoriatic patients who are under long-term treatment. Persistent abnormalities in liver function tests may precede appearance of fibrosis or cirrhosis in the rheumatoid arthritis population [see Warnings and Precautions (5.1)].
5. Methotrexate-induced lung disease, including acute or chronic interstitial pneumonitis, is a potentially dangerous lesion, which may occur acutely at any time during therapy and has been reported at low doses. It is not always fully reversible and fatalities have been reported. Pulmonary symptoms (especially a dry, nonproductive cough) may require interruption of treatment and careful investigation [see Warnings and Precautions (5.1)].
6. Diarrhea and ulcerative stomatitis require interruption of therapy: otherwise, hemorrhagic enteritis and death from intestinal perforation may occur [see Warnings and Precautions (5.1)].
7. Malignant lymphomas, which may regress following withdrawal of methotrexate, may occur in patients receiving low-dose methotrexate and, thus, may not require cytotoxic treatment. Discontinue Otrexup first and, if the lymphoma does not regress, appropriate treatment should be instituted [see Warnings and Precautions (5.8)].
8. Like other cytotoxic drugs, methotrexate may induce “tumor lysis syndrome” in patients with rapidly growing tumors [see Warnings and Precautions (5.9)].
9. Severe, occasionally fatal, skin reactions have been reported following single or multiple doses of methotrexate. Reactions have occurred within days of oral, intramuscular, intravenous, or intrathecal methotrexate administration. Recovery has been reported with discontinuation of therapy [see Warnings and Precautions (5.1)].
10. Potentially fatal opportunistic infections, especially Pneumocystis jiroveci pneumonia, may occur with methotrexate therapy [see Warnings and Precautions (5.1)].
11. Methotrexate given concomitantly with radiotherapy may increase the risk of soft tissue necrosis and osteonecrosis [see Warnings and Precautions (5.10)].
-
1 INDICATIONS AND USAGE
1.1 Rheumatoid Arthritis including Polyarticular Juvenile Idiopathic Arthritis
Otrexup is indicated in the management of selected adults with severe, active rheumatoid arthritis (RA) (ACR criteria), or children with active polyarticular juvenile idiopathic arthritis (pJIA), who have had an insufficient therapeutic response to, or are intolerant of, an adequate trial of first-line therapy including full dose non-steroidal anti-inflammatory agents (NSAIDs).
1.2 Psoriasis
Otrexup is indicated in adults for the symptomatic control of severe, recalcitrant, disabling psoriasis that is not adequately responsive to other forms of therapy, but only when the diagnosis has been established, as by biopsy and/or after dermatologic consultation. It is important to ensure that a psoriasis “flare” is not due to an undiagnosed concomitant disease affecting immune responses.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
Otrexup is a single-dose auto-injector for once-weekly subcutaneous use only [see Warnings and Precautions (5.5)]. Administer Otrexup in the abdomen or the thigh. Otrexup is available in the following dosage strengths: 10, 12.5, 15, 17.5, 20, 22.5 and 25 mg. Use another formulation of methotrexate for alternative dosing in patients who require oral, intramuscular, intravenous, intra-arterial, or intrathecal dosing, doses less than 10 mg per week, doses more than 25 mg per week, high-dose regimens, or dose adjustments between the available doses.
2.2 Rheumatoid Arthritis including Polyarticular Juvenile Idiopathic Arthritis
Recommended starting dose of methotrexate:
Adult RA: 7.5 mg once weekly.
pJIA: 10 mg/m2 once weekly.
For patients switching from oral methotrexate to Otrexup, consider any differences in bioavailability between oral and subcutaneously administered methotrexate [see Clinical Pharmacology (12.3)].
Dosages may be adjusted gradually to achieve an optimal response. Limited experience shows a significant increase in the incidence and severity of serious toxic reactions, especially bone marrow suppression, at doses greater than 20 mg/wk in adults. Although there is experience with doses up to 30 mg/m2/wk in children, there are too few published data to assess how doses over 20 mg/m2/wk might affect the risk of serious toxicity in children. Experience does suggest, however, that children receiving 20 to 30 mg/m2/wk (0.65 to 1.0 mg/kg/wk) may have better absorption and fewer gastrointestinal side effects if methotrexate is administered either intramuscularly or subcutaneously.
Therapeutic response usually begins within 3 to 6 weeks and the patient may continue to improve for another 12 weeks or more.
The optimal duration of therapy is unknown. Limited data available from long-term studies in adults indicate that the initial clinical improvement is maintained for at least two years with continued therapy. When methotrexate is discontinued, the arthritis usually worsens within 3 to 6 weeks.
The patient should be fully informed of the risks involved and should be under constant supervision of the physician. Assessment of hematologic, hepatic, renal, and pulmonary function should be made by history, physical examination, and laboratory tests before beginning, periodically during, and before reinstituting Otrexup therapy [see Warnings and Precautions (5.4)]. Females of childbearing potential should not be started on Otrexup until pregnancy is excluded [see Contraindications (4) and Warnings and Precautions (5.2)]
All schedules should be continually tailored to the individual patient. An initial test dose may be given prior to the regular dosing schedule to detect any extreme sensitivity to adverse effects.
Maximal myelosuppression usually occurs in seven to ten days.
2.3 Psoriasis
Recommended starting dose of methotrexate:
Psoriasis: single weekly oral, intramuscular, subcutaneous, or intravenous doses of 10-25 mg.
For patients switching from oral methotrexate to Otrexup, consider any differences in bioavailability between oral and subcutaneously administered methotrexate [see Clinical Pharmacology (12.3)].
Dosage may be gradually adjusted to achieve optimal clinical response; 30 mg/week should not ordinarily be exceeded. Once optimal clinical response has been achieved, the dosage should be reduced to the lowest possible amount of drug and to the longest possible rest period. The use of Otrexup may permit the return to conventional topical therapy, which should be encouraged.
2.4 Administration and Handling
Otrexup is an auto-injector intended for subcutaneous use under the guidance and supervision of a physician.
Patients may self-inject with Otrexup if a physician determines that it is appropriate, if they have received proper training in how to prepare and administer the correct dose, and if they receive medical follow-up, as necessary. A trainer device is available for training purposes.
Visually inspect Otrexup for particulate matter and discoloration prior to administration. Do not use Otrexup if the seal is broken.
Handle and dispose of Otrexup consistent with recommendations for handling and disposal of cytotoxic drugs1.
-
3 DOSAGE FORMS AND STRENGTHS
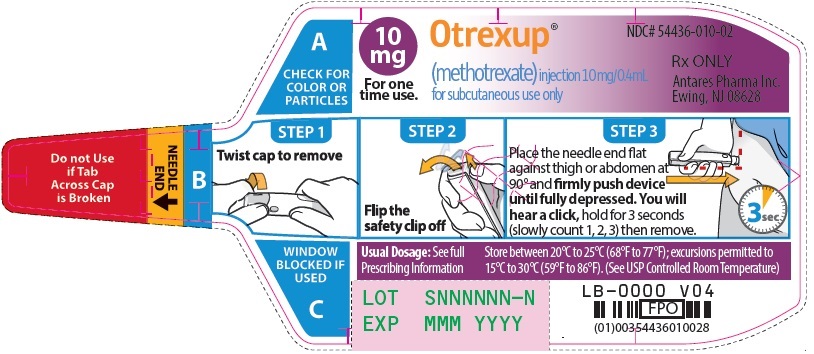
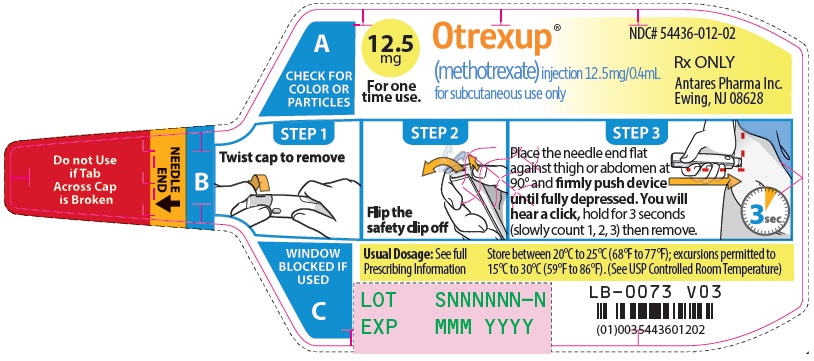
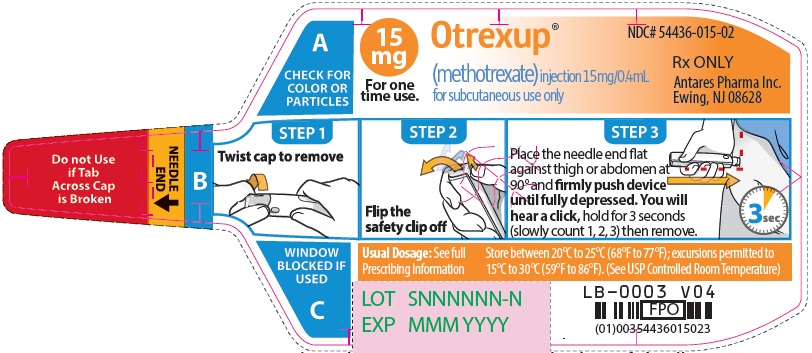
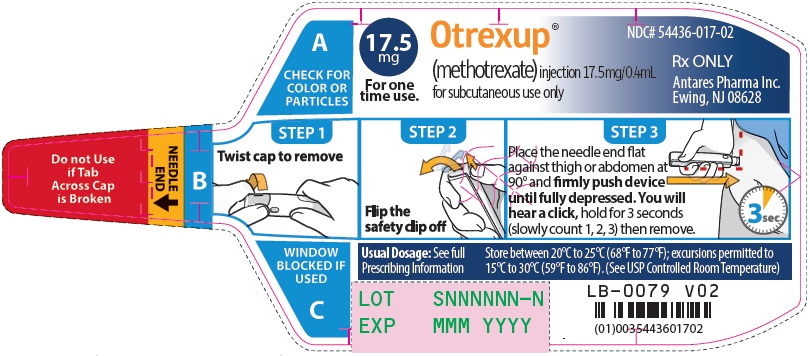
Otrexup is an injection available as an autoinjector that administers a single 0.4 mL dose of methotrexate solution in the following dosage strengths:
- 10 mg/0.4 mL methotrexate
- 12.5 mg/0.4 mL methotrexate
- 15 mg/0.4 mL methotrexate
- 17.5 mg/0.4 mL methotrexate
- 20 mg/0.4 mL methotrexate
- 22.5 mg/0.4 mL methotrexate
- 25 mg/0.4 mL methotrexate
-
4 CONTRAINDICATIONS
Otrexup is contraindicated in the following:
• Pregnancy
Otrexup can cause embryo-fetal toxicity and fetal death when administered during pregnancy [see Warnings and Precautions (5.2) and Use in Specific Populations (8.1)].
• Alcoholism or Liver Disease
Patients with alcoholism, alcoholic liver disease or other chronic liver disease [see Warnings and Precautions (5.1)].
• Immunodeficiency Syndromes
Patients who have overt or laboratory evidence of immunodeficiency syndromes [see Warnings and Precautions (5.1)].
• Preexisting Blood Dyscrasias
Patients who have preexisting blood dyscrasias, such as bone marrow hypoplasia, leukopenia, thrombocytopenia, or significant anemia [see Warnings and Precautions (5.1)].
• Hypersensitivity
Patients with a known hypersensitivity to methotrexate. Severe hypersensitivity reactions have been observed with methotrexate use [see Warnings and Precautions (5.1) and Adverse Reactions (6.1 and 6.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Organ System Toxicity
Otrexup should be used only by physicians whose knowledge and experience include the use of antimetabolite therapy. Because of the possibility of serious toxic reactions (which can be fatal), Otrexup should be used only in patients with psoriasis or rheumatoid arthritis with severe, recalcitrant, disabling disease which is not adequately responsive to other forms of therapy.
Deaths have been reported with the use of methotrexate in the treatment of malignancy, psoriasis, and rheumatoid arthritis. Patients should be closely monitored for bone marrow, liver, lung and kidney toxicities.
Otrexup has the potential for serious toxicity. Toxic effects may be related in frequency and severity to dose or frequency of administration but have been seen at all doses. Because they can occur at any time during therapy, it is necessary to follow patients on Otrexup closely. Most adverse reactions are reversible if detected early. When such reactions do occur, the drug should be reduced in dosage or discontinued and appropriate corrective measures should be taken. If necessary, this could include the use of leucovorin calcium and/or acute, intermittent hemodialysis with a high-flux dialyzer [see Overdosage (10)]. If Otrexup therapy is reinstituted, it should be carried out with caution, with adequate consideration of further need for the drug and increased alertness as to possible recurrence of toxicity. The clinical pharmacology of methotrexate has not been well studied in older individuals. Due to diminished hepatic and renal function as well as decreased folate stores in this population, relatively low doses should be considered, and these patients should be closely monitored for early signs of toxicity [see Use in Specific Populations (8.5)].
Gastrointestinal:
Diarrhea and ulcerative stomatitis require interruption of therapy: otherwise, hemorrhagic enteritis and death from intestinal perforation may occur.
If vomiting, diarrhea, or stomatitis occur, which may result in dehydration, Otrexup should be discontinued until recovery occurs. Otrexup should be used with extreme caution in the presence of peptic ulcer disease or ulcerative colitis.
Unexpectedly severe (sometimes fatal) gastrointestinal toxicity has been reported with concomitant administration of methotrexate (usually in high dosage) along with some nonsteroidal anti-inflammatory drugs (NSAIDs) [see Drug Interactions (7.1)].
Hematologic:
Otrexup can suppress hematopoiesis and cause anemia, aplastic anemia, pancytopenia, leukopenia, neutropenia, and/or thrombocytopenia. In patients with preexisting hematopoietic impairment, Otrexup should be used with caution, if at all. In controlled clinical trials conducted with another formulation of methotrexate in rheumatoid arthritis (n=128), leukopenia (WBC <3000/mm3) was seen in 2 patients, thrombocytopenia (platelets <100,000/mm3) in 6 patients, and pancytopenia in 2 patients.
Otrexup should be stopped immediately if there is a significant drop in blood counts. Patients with profound granulocytopenia and fever should be evaluated immediately and usually require parenteral broad-spectrum antibiotic therapy.
Unexpectedly severe (sometimes fatal) bone marrow suppression and aplastic anemia have been reported with concomitant administration of methotrexate (usually in high dosage) along with some nonsteroidal anti-inflammatory drugs (NSAIDs) [see Drug Interactions (7.1)].
Hepatic:
Otrexup has the potential for acute (elevated transaminases) and chronic (fibrosis and cirrhosis) hepatotoxicity. Chronic toxicity is potentially fatal; it generally has occurred after prolonged use (generally two years or more) and after a total dose of at least 1.5 grams. In studies in psoriatic patients, hepatotoxicity appeared to be a function of total cumulative dose and appeared to be enhanced by alcoholism, obesity, diabetes and advanced age. An accurate incidence rate has not been determined; the rate of progression and reversibility of lesions is not known. Special caution is indicated in the presence of preexisting liver damage or impaired hepatic function.
In psoriasis, liver function tests, including serum albumin, should be performed periodically prior to dosing but are often normal in the face of developing fibrosis or cirrhosis. These lesions may be detectable only by biopsy. The usual recommendation is to obtain a liver biopsy at 1) pretherapy or shortly after initiation of therapy (2 to 4 months), 2) a total cumulative dose of 1.5 grams, and 3) after each additional 1.0 to 1.5 grams. Moderate fibrosis or any cirrhosis normally leads to discontinuation of the drug; mild fibrosis normally suggests a repeat biopsy in 6 months.
Milder histologic findings such as fatty change and low grade portal inflammation, are relatively common pretherapy. Although these mild changes are usually not a reason to avoid or discontinue Otrexup therapy, the drug should be used with caution.
In rheumatoid arthritis, age at first use of methotrexate and duration of therapy have been reported as risk factors for hepatotoxicity; other risk factors, similar to those observed in psoriasis, may be present in rheumatoid arthritis but have not been confirmed to date. Persistent abnormalities in liver function tests may precede appearance of fibrosis or cirrhosis in this population. There is a combined reported experience in 217 rheumatoid arthritis patients with liver biopsies both before and during treatment (after a cumulative dose of at least 1.5 g) and in 714 patients with a biopsy only during treatment. There are 64 (7%) cases of fibrosis and 1 (0.1%) case of cirrhosis. Of the 64 cases of fibrosis, 60 were deemed mild. The reticulin stain is more sensitive for early fibrosis and its use may increase these figures. It is unknown whether even longer use will increase these risks.
Liver function tests should be performed at baseline at 4 to 8 week intervals in patients receiving Otrexup for rheumatoid arthritis. Pretreatment liver biopsy should be performed for patients with a history of excessive alcohol consumption, persistently abnormal baseline liver function test values or chronic hepatitis B or C infection. During therapy, liver biopsy should be performed if there are persistent liver function test abnormalities or there is a decrease in serum albumin below the normal range (in the setting of well controlled rheumatoid arthritis).
If the results of a liver biopsy show mild changes (Roenigk, grades I, II, IIIa), Otrexup may be continued and the patient monitored as per recommendations listed above. Otrexup should be discontinued in any patient who displays persistently abnormal liver function tests and refuses liver biopsy or in any patient whose liver biopsy shows moderate to severe changes (Roenigk grade IIIb or IV).
Infection or Immunologic States:
Otrexup should be used with extreme caution in the presence of active infection, and is contraindicated in patients with overt or laboratory evidence of immunodeficiency syndromes.
Immunization may be ineffective when given during Otrexup therapy. Immunization with live virus vaccines is generally not recommended. There have been reports of disseminated vaccinia infections after smallpox immunizations in patients receiving methotrexate therapy. Hypogammaglobulinemia has been reported rarely.
Potentially fatal opportunistic infections, especially Pneumocystis jiroveci pneumonia, may occur with Otrexup therapy. When a patient presents with pulmonary symptoms, the possibility of Pneumocystis jiroveci pneumonia should be considered.
Neurologic:
There have been reports of leukoencephalopathy following intravenous administration of methotrexate to patients who have had craniospinal irradiation. Serious neurotoxicity, frequently manifested as generalized or focal seizures, has been reported with unexpectedly increased frequency among pediatric patients with acute lymphoblastic leukemia who were treated with intermediate-dose intravenous methotrexate (1 gm/m2). Symptomatic patients were commonly noted to have leukoencephalopathy and/or microangiopathic calcifications on diagnostic imaging studies. Chronic leukoencephalopathy has also been reported in patients who received repeated doses of high-dose methotrexate with leucovorin rescue even without cranial irradiation.
Discontinuation of methotrexate does not always result in complete recovery. A transient acute neurologic syndrome has been observed in patients treated with high dose regimens. Manifestations of this stroke-like encephalopathy may include confusion, hemiparesis, transient blindness, seizures and coma. The exact cause is unknown. After the intrathecal use of methotrexate, the central nervous system toxicity which may occur can be classified as follows: acute chemical arachnoiditis manifested by such symptoms as headache, back pain, nuchal rigidity, and fever; sub-acute myelopathy characterized by paraparesis/paraplegia associated with involvement with one or more spinal nerve roots; chronic leukoencephalopathy manifested by confusion, irritability, somnolence, ataxia, dementia, seizures and coma. This condition can be progressive and even fatal.
Pulmonary:
Methotrexate-induced lung disease, including acute or chronic interstitial pneumonitis, is a potentially dangerous lesion, which may occur acutely at any time during therapy and has been reported at low doses. It is not always fully reversible and fatalities have been reported.
Pulmonary symptoms (especially a dry nonproductive cough) or a non-specific pneumonitis occurring during Otrexup therapy may be indicative of a potentially dangerous lesion and require interruption of treatment and careful investigation. Although clinically variable, the typical patient with methotrexate induced lung disease presents with fever, cough, dyspnea, hypoxemia, and an infiltrate on chest X-ray; infection (including pneumonia) needs to be excluded. This lesion can occur at all dosages.
Renal:
Otrexup may cause renal damage that may lead to acute renal failure. High doses of methotrexate used in the treatment of osteosarcoma may cause renal damage leading to acute renal failure. Nephrotoxicity is due primarily to the precipitation of methotrexate and 7- hydroxymethotrexate in the renal tubules. Close attention to renal function including adequate hydration, urine alkalinization and measurement of serum methotrexate and creatinine levels are essential for safe administration.
Skin:
Severe, occasionally fatal, dermatologic reactions, including toxic epidermal necrolysis, Stevens-Johnson syndrome, exfoliative dermatitis, skin necrosis, and erythema multiforme, have been reported in children and adults, within days of oral, intramuscular, intravenous, or intrathecal methotrexate administration. Reactions were noted after single or multiple low, intermediate, or high doses of methotrexate in patients with neoplastic and non-neoplastic diseases.
Lesions of psoriasis may be aggravated by concomitant exposure to ultraviolet radiation.
Radiation dermatitis and sunburn may be “recalled” by the use of methotrexate.
Other precautions:
Otrexup should be used with extreme caution in the presence of debility.
Methotrexate exits slowly from third space compartments (e.g., pleural effusions or ascites). This results in a prolonged terminal plasma half-life and unexpected toxicity. In patients with significant third space accumulations, it is advisable to evacuate the fluid before treatment and to monitor plasma methotrexate levels.
5.2 Embryo-Fetal Toxicity
Based on published reports and methotrexate’s mechanism of action, methotrexate can cause embryo-fetal toxicity and fetal death when administered to a pregnant woman. In pregnant women Otrexup is contraindicated. Verify pregnancy status in females of reproductive potential prior to initiating Otrexup.
Advise females of reproductive potential to use effective contraception during treatment with Otrexup and for 6 months after the final dose. Advise males of reproductive potential to use effective contraception during Otrexup treatment and for at least 3 months after the final dose [see Contraindications (4), Use in Specific Populations (8.1, 8.3), Clinical Pharmacology (12.1)].
5.3 Effects on Reproduction
Based on published reports, methotrexate can cause impairment of fertility, oligospermia, and menstrual dysfunction. It is not known if the infertility is reversible in affected patients. Discuss the risk of effects on reproduction with female and male patients of reproductive potential. [see Use in Specific Populations (8.3)].
5.4 Laboratory Tests
Patients undergoing Otrexup therapy should be closely monitored so that toxic effects are detected promptly. Baseline assessment should include a complete blood count with differential and platelet counts, hepatic enzymes, renal function tests and a chest X-ray.
During therapy, monitoring of these parameters is recommended: hematology at least monthly, renal function and liver function every 1 to 2 months [see Warnings and Precautions (5.1)].
During initial or changing doses, or during periods of increased risk of elevated methotrexate blood levels (e.g., dehydration), more frequent monitoring may also be indicated.
Liver Function Tests
Transient liver function test abnormalities are observed frequently after methotrexate administration and are usually not cause for modification of methotrexate therapy. Persistent liver function test abnormalities, and/or depression of serum albumin may be indicators of serious liver toxicity and require evaluation [see Warnings and Precautions (5.1)].
A relationship between abnormal liver function tests and fibrosis or cirrhosis of the liver has not been established for patients with psoriasis. Persistent abnormalities in liver function tests may precede appearance of fibrosis or cirrhosis in the rheumatoid arthritis population.
Pulmonary Function Tests
Pulmonary function tests may be useful if methotrexate-induced lung disease is suspected, especially if baseline measurements are available [see Warnings and Precautions (5.1)].
5.5 Risks from Improper Dosing
Both the physician and pharmacist should emphasize to the patient that Otrexup is administered weekly and that mistaken daily use has led to fatal toxicity [see Dosage and Administration (2)].
5.6 Patients with Impaired Renal Function, Ascites, or Pleural Effusions
Methotrexate elimination is reduced in patients with impaired renal function, ascites, or pleural effusions. Such patients require especially careful monitoring for toxicity and require dose reduction or, in some cases, discontinuation of Otrexup administration.
5.7 Dizziness and Fatigue
Adverse reactions, such as dizziness and fatigue, may affect the ability to drive or operate machinery.
5.8 Malignant Lymphomas
Non-Hodgkin’s lymphoma and other tumors have been reported in patients receiving low-dose oral methotrexate. However, there have been instances of malignant lymphoma arising during treatment with low-dose oral methotrexate, which have regressed completely following withdrawal of methotrexate, without requiring active anti-lymphoma treatment. Discontinue Otrexup first and, if the lymphoma does not regress, appropriate treatment should be instituted.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling.
- Organ System Toxicity [see Warnings and Precautions (5.1)]
- Embryo-Fetal Toxicity [see Warnings and Precautions (5.2)]
- Effects on Reproduction [see Warnings and Precautions (5.3)]
- Malignant Lymphomas [see Warnings and Precautions (5.8)]
The most frequently reported adverse reactions include ulcerative stomatitis, leukopenia, nausea, and abdominal distress. Other frequently reported adverse reactions are malaise, undue fatigue, chills and fever, dizziness and decreased resistance to infection.
6.1 Clinical Trials Experience
This section provides a summary of adverse reactions reported in subjects in clinical studies conducted with Otrexup as well as with methotrexate injection and oral methotrexate.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug, and may not reflect the rates observed in practice.
Rheumatoid Arthritis
The approximate incidences of methotrexate-attributed (i.e. placebo rate subtracted) adverse reactions in 12 to 18 week double-blind studies of patients (n=128) with rheumatoid arthritis treated with low-dose oral (7.5 to 15 mg/week) pulse methotrexate, are listed below. Virtually all of these patients were on concomitant nonsteroidal anti-inflammatory drugs and some were also taking low dosages of corticosteroids. Hepatic histology was not examined in these short-term studies.
Incidence greater than 10%: Elevated liver function tests 15%, nausea/vomiting 10%.
Incidence 3% to 10%: Stomatitis, thrombocytopenia (platelet count less than 100,000/mm3).
Incidence 1% to 3%: Rash/pruritis/dermatitis, diarrhea, alopecia, leukopenia (WBC less than 3000/mm3), pancytopenia, dizziness.
Two other controlled trials of patients (n=680) with Rheumatoid Arthritis on 7.5 mg to 15 mg/wk oral doses showed an incidence of interstitial pneumonitis of 1%.
Other less common reactions included decreased hematocrit, headache, upper respiratory infection, anorexia, arthralgias, chest pain, coughing, dysuria, eye discomfort, epistaxis, fever, infection, sweating, tinnitus, and vaginal discharge.
Polyarticular Juvenile Idiopathic Arthritis
The approximate incidences of adverse reactions reported in pediatric patients with pJIA treated with oral, weekly doses of methotrexate (5 to 20 mg/m2/wk or 0.1 to 0.65 mg/kg/wk) were as follows (virtually all patients were receiving concomitant nonsteroidal anti-inflammatory drugs, and some also were taking low doses of corticosteroids): elevated liver function tests, 14%; gastrointestinal reactions (e.g., nausea, vomiting, diarrhea), 11%; stomatitis, 2%; leukopenia, 2%; headache, 1.2%; alopecia, 0.5%; dizziness, 0.2%; and rash, 0.2%. Although there is experience with dosing up to 30 mg/m2/wk in pJIA, the published data for doses above 20 mg/m2/wk are too limited to provide reliable estimates of adverse reaction rates.
Psoriasis
There are two literature reports (Roenigk, 1969, and Nyfors, 1978) describing large series (n=204, 248) of psoriasis patients treated with methotrexate. Dosages ranged up to 25 mg per week and treatment was administered for up to four years. With the exception of alopecia, photosensitivity, and “burning of skin lesions” (each 3% to 10%), the adverse reaction rates in these reports were very similar to those in the rheumatoid arthritis studies. Rarely, painful plaque erosions may appear (Pearce, HP and Wilson, BB: Am Acad Dermatol 35: 835-838, 1996).
6.2 Other Adverse Reactions
Other adverse reactions that have been reported with methotrexate in oncology, RA, pJIA, and psoriasis patients are listed below by organ system.
Alimentary System: gingivitis, pharyngitis, stomatitis, anorexia, nausea, vomiting, diarrhea, hematemesis, melena, gastrointestinal ulceration and bleeding, enteritis, pancreatitis.
Blood and Lymphatic System Disorders: suppressed hematopoiesis, anemia, aplastic anemia, pancytopenia, leukopenia, neutropenia, thrombocytopenia, agranulocytosis, eosinophilia, lymphadenopathy and lymphoproliferative disorders (including reversible). Hypogammaglobulinemia has been reported rarely.
Cardiovascular: pericarditis, pericardial effusion, hypotension, and thromboembolic events (including arterial thrombosis, cerebral thrombosis, deep vein thrombosis, retinal vein thrombosis, thrombophlebitis, and pulmonary embolus).
Central Nervous System: headaches, drowsiness, blurred vision, transient blindness, speech impairment including dysarthria and aphasia, hemiparesis, paresis and convulsions have also occurred following administration of methotrexate. Following low doses, there have been occasional reports of transient subtle cognitive dysfunction, mood alteration or unusual cranial sensations, leukoencephalopathy, or encephalopathy.
Hepatobiliary Disorders: hepatotoxicity, acute hepatitis, chronic fibrosis and cirrhosis, hepatic failure, decrease in serum albumin, liver enzyme elevations.
Infection: There have been case reports of sometimes fatal opportunistic infections in patients receiving methotrexate therapy for neoplastic and non-neoplastic diseases. Pneumocystis jiroveci pneumonia was the most common opportunistic infection. There have also been reports of infections, pneumonia, Cytomegalovirus infection, including cytomegaloviral pneumonia, sepsis, fatal sepsis, nocardiosis; histoplasmosis, cryptococcosis, Herpes zoster, Herpes simplex hepatitis, and disseminated Herpes simplex.
Musculoskeletal System: stress fracture.
Ophthalmic: conjunctivitis, serious visual changes of unknown etiology.
Pulmonary System: respiratory fibrosis, respiratory failure, alveolitis, interstitial pneumonitis deaths have been reported, and chronic interstitial obstructive pulmonary disease has occasionally occurred.
Skin: erythematous rashes, pruritus, urticaria, photosensitivity, pigmentary changes, alopecia, ecchymosis, telangiectasia, acne, furunculosis, erythema multiforme, toxic epidermal necrolysis, Stevens-Johnson syndrome, skin necrosis, skin ulceration and exfoliative dermatitis.
Urogenital System: severe nephropathy or renal failure, azotemia, cystitis, hematuria, proteinuria; defective oogenesis or spermatogenesis, transient oligospermia, menstrual dysfunction, vaginal discharge, and gynecomastia; infertility, abortion, fetal death, fetal defects.
Other rarer reactions related to or attributed to the use of methotrexate such as nodulosis, vasculitis, arthralgia/myalgia, loss of libido/ impotence, diabetes, osteoporosis, sudden death, lymphoma, including reversible lymphomas, tumor lysis syndrome, soft tissue necrosis and osteonecrosis. Anaphylactoid reactions have been reported.
-
7 DRUG INTERACTIONS
7.1 Aspirin, Nonsteroidal Anti-Inflammatory Drugs, and Steroids
Nonsteroidal anti-inflammatory drugs (NSAIDs) should not be administered prior to or concomitantly with the high doses of methotrexate, such as used in the treatment of osteosarcoma. Concomitant administration of some NSAIDs with high dose methotrexate therapy has been reported to elevate and prolong serum methotrexate levels, resulting in deaths from severe hematologic and gastrointestinal toxicity [see Warnings and Precautions (5.1)].
Caution should be used when NSAIDs and salicylates are administered concomitantly with lower doses of methotrexate, including Otrexup. These drugs have been reported to reduce the tubular secretion of methotrexate in an animal model and may enhance its toxicity.
Despite the potential interactions, studies of methotrexate in patients with rheumatoid arthritis have usually included concurrent use of constant dosage regimens of NSAIDs, without apparent problems. It should be appreciated, however, that the doses used in rheumatoid arthritis (7.5 to 15 mg/week) are somewhat lower than those used in psoriasis and that larger doses could lead to unexpected toxicity. Aspirin, NSAIDs, and/or low dose steroids may be continued, although the possibility of increased toxicity with concomitant use of NSAIDs including salicylates has not been fully explored. Steroids may be reduced gradually in patients who respond to methotrexate.
7.2 Proton Pump Inhibitors (PPIs)
Use caution if high-dose methotrexate is administered to patients receiving proton pump inhibitor (PPI) therapy. Case reports and published population pharmacokinetic studies suggest that concomitant use of some PPIs, such as omeprazole, esomeprazole, and pantoprazole, with methotrexate (primarily at high dose), may elevate and prolong serum levels of methotrexate and/or its metabolite hydroxymethotrexate, possibly leading to methotrexate toxicities. In two of these cases, delayed methotrexate elimination was observed when high-dose methotrexate was co-administered with PPIs, but was not observed when methotrexate was co-administered with ranitidine. However, no formal drug interaction studies of methotrexate with ranitidine have been conducted.
7.3 Oral Antibiotics
Oral antibiotics such as tetracycline, chloramphenicol, and nonabsorbable broad spectrum antibiotics, may decrease intestinal absorption of methotrexate or interfere with the enterohepatic circulation by inhibiting bowel flora and suppressing metabolism of the drug by bacteria.
Penicillins may reduce the renal clearance of methotrexate; increased serum concentrations of methotrexate with concomitant hematologic and gastrointestinal toxicity have been observed with high and low dose methotrexate. Use of Otrexup with penicillins should be carefully monitored.
Trimethoprim/sulfamethoxazole has been reported rarely to increase bone marrow suppression in patients receiving methotrexate, probably by decreased tubular secretion and/or an additive antifolate effect.
7.4 Hepatotoxins
The potential for increased hepatotoxicity when methotrexate is administered with other hepatotoxic agents has not been evaluated. However, hepatotoxicity has been reported in such cases. Therefore, patients receiving concomitant therapy with Otrexup and other potential hepatotoxins (e.g., azathioprine, retinoids, and sulfasalazine) should be closely monitored for possible increased risk of hepatotoxicity.
7.5 Theophylline
Methotrexate may decrease the clearance of theophylline; theophylline levels should be monitored when used concurrently with Otrexup.
7.6 Folic Acid and Antifolates
Vitamin preparations containing folic acid or its derivatives may decrease responses to systemically administered methotrexate. Preliminary animal and human studies have shown that small quantities of intravenously administered leucovorin enter the CSF primarily as 5-methyltetrahydrofolate and, in humans, remain 1 to 3 orders of magnitude lower than the usual methotrexate concentrations following intrathecal administration. However, high doses of leucovorin may reduce the efficacy of intrathecally administered methotrexate. Folate deficiency states may increase methotrexate toxicity.
Trimethoprim/sulfamethoxazole has been reported rarely to increase bone marrow suppression in patients receiving methotrexate, probably by decreased tubular secretion and/or an additive antifolate effect.
7.7 Mercaptopurine
Methotrexate increases the plasma levels of mercaptopurine. The combination of Otrexup and mercaptopurine may therefore require dose adjustment.
7.8 Nitrous Oxide
The use of nitrous oxide anesthesia potentiates the effect of methotrexate on folate dependent metabolic pathways, resulting in the potential for increased toxicity. Avoid concomitant nitrous oxide anesthesia in patients receiving methotrexate.
7.9 Other Drugs
Methotrexate is partially bound to serum albumin, and toxicity may be increased because of displacement by certain drugs, such as salicylates, phenylbutazone, phenytoin, and sulfonamides.
Renal tubular transport is also diminished by probenecid; use of Otrexup with this drug should be carefully monitored.
Combined use of methotrexate with gold, penicillamine, hydroxychloroquine, sulfasalazine, or cytotoxic agents, has not been studied and may increase the incidence of adverse effects.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on published reports and methotrexate’s mechanism of action, methotrexate can cause embryo-fetal toxicity and fetal death when administered to a pregnant woman [see Data and Clinical Pharmacology (12.1)]. In pregnant women with non-malignant disease, Otrexup is contraindicated. Consider the benefits and risks of Otrexup and risks to the fetus when prescribing Otrexup to a pregnant patient. There are no animal data that meet current standards for nonclinical developmental toxicity studies.
The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Human Data
Published data from cases, literature reviews, and observational studies report that methotrexate exposure during pregnancy is associated with an increased risk of embryo-fetal toxicity and fetal death. Methotrexate exposure during the first trimester of pregnancy is associated with an increased incidence of spontaneous abortions and multiple adverse developmental outcomes, including skull anomalies, facial dysmorphism, central nervous system abnormalities, limb abnormalities, and sometimes cardiac anomalies and intellectual impairment. Adverse outcomes associated with exposure during second and third trimesters of pregnancy include intrauterine growth restriction and functional abnormalities. Because methotrexate is widely distributed and persists in the body for a prolonged period, there is a potential risk to the fetus from preconception methotrexate exposure.
A prospective multicenter study evaluated pregnancy outcomes in women taking methotrexate less than or equal to 30 mg/week after conception. The rate of miscarriage in pregnant women exposed to methotrexate was 42.5% (95% confidence interval [95% CI] 29.2-58.7), which was higher than in unexposed patients with autoimmune disease (22.5%, 95% CI 16.8-29.7) and unexposed patients with non-autoimmune disease (17.3%, 95% CI 13-22.8). Of the live births, the rate of major birth defects in pregnant women exposed to methotrexate after conception was higher than in unexposed patients with autoimmune disease (adjusted odds ratio (OR) 1.8 [95% CI 0.6-5.7]) and unexposed patients with non-autoimmune disease (adjusted OR 3.1 [95% CI 1.03-9.5]). Major birth defects associated with pregnancies exposed to methotrexate after conception were not always consistent with methotrexate-associated adverse developmental outcomes.
8.2 Lactation
Risk Summary
Limited published literature report the presence of methotrexate in human milk in low amounts following oral methotrexate administration, with the highest breast milk to plasma concentration ratio reported to be 0.08:1. No information is available on the effects of methotrexate on a breastfed infant or on milk production. Because of the potential for serious adverse reactions including myelosuppression, from methotrexate in breastfed infants, advise women not to breastfeed during treatment with Otrexup and for one week after the final dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating Otrexup.
Contraception
Females
Otrexup can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during and for 6 months after the final dose of Otrexup.
Males
Methotrexate can cause chromosomal damage to sperm cells. Advise males with female partners of reproductive potential to use effective contraception during and for at least 3 months after the final dose of Otrexup.
Infertility
Females
Based on published reports of female infertility after treatment with methotrexate, advise females of reproductive potential that Otrexup can cause impairment of fertility and menstrual dysfunction during and after cessation of therapy. It is not known if the infertility may be reversed in all affected females.
Males
Based on published reports of male infertility after treatment with methotrexate, advise males of reproductive potential that Otrexup can cause oligospermia or infertility during and after cessation of therapy. It is not known if the infertility may be reversed in all affected males.
8.4 Pediatric Use
The safety and effectiveness of methotrexate, including Otrexup, have not been established in pediatric patients with psoriasis.
The safety and effectiveness of Otrexup have not been established in pediatric patients with neoplastic diseases.
The safety and effectiveness of methotrexate have been established in pediatric patients with polyarticular juvenile idiopathic arthritis [see Clinical Studies (14.2)].
Published clinical studies evaluating the use of methotrexate in children and adolescents (i.e., patients 2 to 16 years of age) with pJIA demonstrated safety comparable to that observed in adults with rheumatoid arthritis [see Adverse Reactions (6.1)].
Otrexup does not contain a preservative. However, methotrexate injectable formulations containing the preservative benzyl alcohol are not recommended for use in neonates. There have been reports of fatal ‘gasping syndrome’ in neonates (children less than one month of age) following the administrations of intravenous solutions containing the preservative benzyl alcohol. Symptoms include a striking onset of gasping respiration, hypotension, bradycardia, and cardiovascular collapse.
Serious neurotoxicity, frequently manifested as generalized or focal seizures, has been reported with unexpectedly increased frequency among pediatric patients with acute lymphoblastic leukemia who were treated with intermediate-dose intravenous methotrexate (1 gm/m2) [see Warnings and Precautions (5.1)].
8.5 Geriatric Use
Clinical studies of methotrexate did not include sufficient numbers of subjects age 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious reflecting the greater frequency of decreased hepatic and renal function, decreased folate stores, concomitant disease or other drug therapy (i.e., that interfere with renal function, methotrexate or folate metabolism) in this population [see Warnings and Precautions (5.1), Drug Interactions (7.7) and Use in Specific Populations (8.6)]. Since decline in renal function may be associated with increases in adverse reactions and serum creatinine measurements may over estimate renal function in the elderly, more accurate methods (i.e., creatinine clearance) should be considered. Serum methotrexate levels may also be helpful. Elderly patients should be closely monitored for early signs of hepatic, bone marrow and renal toxicity. In chronic use situations, certain toxicities may be reduced by folate supplementation. Post-marketing experience suggests that the occurrence of bone marrow suppression, thrombocytopenia, and pneumonitis may increase with age [see Warnings and Precautions (5.1)].
8.6 Renal Impairment
Methotrexate elimination is reduced in patients with impaired renal function. Such patients require especially careful monitoring for toxicity and require dose reduction or, in some cases, discontinuation of Otrexup administration.
8.7 Hepatic Impairment
The effect of hepatic impairment on methotrexate pharmacokinetics has not been studied. Otrexup is contraindicated in patients with alcoholic liver disease or other chronic liver disease. Patients with obesity, diabetes, hepatic fibrosis or steatohepatitis are at increased risk for hepatic injury and fibrosis secondary to methotrexate, and should be monitored closely [see Warnings and Precautions (5.1)].
-
10 OVERDOSAGE
Leucovorin is indicated to diminish the toxicity and counteract the effect of inadvertently administered overdosages of methotrexate. Leucovorin administration should begin as promptly as possible. As the time interval between methotrexate administration and leucovorin initiation increases, the effectiveness of leucovorin in counteracting toxicity decreases. Monitoring of the serum methotrexate concentration is essential in determining the optimal dose and duration of treatment with leucovorin.
In cases of massive overdosage, hydration and urinary alkalinization may be necessary to prevent the precipitation of methotrexate and/or its metabolites in the renal tubules. Generally speaking, neither hemodialysis nor peritoneal dialysis has been shown to improve methotrexate elimination. However, effective clearance of methotrexate has been reported with acute, intermittent hemodialysis using a high-flux dialyzer (Wall, SM et al: Am J Kidney Dis 28 (6): 846-854, 1996).
Accidental intrathecal overdosage may require intensive systemic support, high-dose systemic leucovorin, alkaline diuresis and rapid CSF drainage and ventriculolumbar perfusion.
In postmarketing experience, overdose with methotrexate has generally occurred with oral and intrathecal administration, although intravenous and intramuscular overdose have also been reported.
Reports of oral overdose often indicate accidental daily administration instead of weekly (single or divided doses). Symptoms commonly reported following oral overdose include those symptoms and signs reported at pharmacologic doses, particularly hematologic and gastrointestinal reaction. For example, leukopenia, thrombocytopenia, anemia, pancytopenia, bone marrow suppression, mucositis, stomatitis, oral ulceration, nausea, vomiting, gastrointestinal ulceration, gastrointestinal bleeding. In some cases, no symptoms were reported.
There have been reports of death following overdose. In these cases, events such as sepsis or septic shock, renal failure, and aplastic anemia were also reported.
Symptoms of intrathecal overdose are generally central nervous system (CNS) symptoms, including headache, nausea and vomiting, seizure or convulsion, and acute toxic encephalopathy. In some cases, no symptoms were reported. There have been reports of death following intrathecal overdose. In these cases, cerebellar herniation associated with increased intracranial pressure, and acute toxic encephalopathy have also been reported.
There are published case reports of intravenous and intrathecal carboxypeptidase G2 treatment to hasten clearance of methotrexate in cases of overdose.
-
11 DESCRIPTION
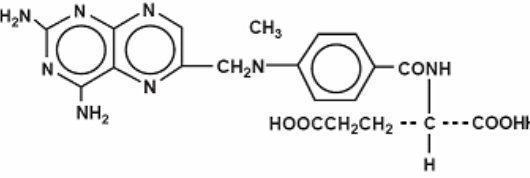
Otrexup contains methotrexate, a folate analog metabolic inhibitor.
Chemically, methotrexate is [N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-Lglutamic acid. The structural formula is:
C20H22N8O5 M.W.= 454.45
Otrexup contains methotrexate in a sterile, preservative-free, unbuffered solution with a 27 gauge ½ inch needle for a single subcutaneous injection. Otrexup solution is yellow in color.
Inactive ingredients include sodium chloride and water for injection, USP. The amounts of sodium chloride vary with the amount of methotrexate.
Amount of methotrexate (mg) per 0.4 mL
10
12.5
15
17.5
20
22.5
25
Amount of sodium chloride (mg) per 0.4 mL
1.96
2.04
1.60
1.48
1.28
0.92
0.56
Hydrochloric acid and additional sodium hydroxide may have been added, if necessary, to adjust the pH to 8.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Methotrexate inhibits dihydrofolic acid reductase. Dihydrofolates must be reduced to tetrahydrofolates by this enzyme before they can be utilized as carriers of one-carbon groups in the synthesis of purine nucleotides and thymidylate. Therefore, methotrexate interferes with DNA synthesis, repair, and cellular replication. Actively proliferating tissues such as malignant cells, bone marrow, fetal cells, buccal and intestinal mucosa, and cells of the urinary bladder are in general more sensitive to this effect of methotrexate.
The mechanism of action in rheumatoid arthritis is unknown; it may affect immune function.
12.2 Pharmacodynamics
Two reports describe in vitro methotrexate inhibition of DNA precursor uptake by stimulated mononuclear cells, and another describes in animal polyarthritis partial correction by methotrexate of spleen cell hyporesponsiveness and suppressed IL 2 production. Other laboratories, however, have been unable to demonstrate similar effects. Clarification of methotrexate’s effect on immune activity and its relation to rheumatoid immunopathogenesis await further studies.
In psoriasis, the rate of production of epithelial cells in the skin is greatly increased over normal skin. This differential in proliferation rates is the basis for the use of methotrexate to control the psoriatic process.
Methotrexate in high doses, followed by leucovorin rescue, is used as a part of the treatment of patients with non-metastatic osteosarcoma. The original rationale for high dose methotrexate therapy was based on the concept of selective rescue of normal tissues by leucovorin. More recent evidence suggests that high dose methotrexate may also overcome methotrexate resistance caused by impaired active transport, decreased affinity of dihydrofolic acid reductase for methotrexate, increased levels of dihydrofolic acid reductase resulting from gene amplification, or decreased polyglutamation of methotrexate. The actual mechanism of action is unknown.
12.3 Pharmacokinetics
Absorption
In adults, oral absorption appears to be dose dependent. Peak serum levels are reached within one to two hours. At doses of 30 mg/m2 or less, methotrexate is generally well absorbed with a mean bioavailability of about 60%. The absorption of doses greater than 80 mg/m2 is significantly less, possibly due to a saturation effect.
In relative bioavailability studies in rheumatoid arthritis patients, systemic exposure of methotrexate was found to be similar between Otrexup and intramuscular or subcutaneous administration of methotrexate injection at the same doses, however systemic exposure of methotrexate was higher with Otrexup as compared to oral administration of methotrexate at the same dose. Bioavailability following oral dosing showed a plateau effect at doses of 15 mg and greater. The systemic exposure of methotrexate from Otrexup at doses of 10, 15, 20, and 25 mg was higher than that of oral methotrexate by 17, 13, 31, and 36%, respectively. Methotrexate systemic absorption from Otrexup was similar when administered into the abdomen or thigh.
In leukemic pediatric patients, oral absorption of methotrexate also appears to be dose dependent and has been reported to vary widely (23% to 95%). A twenty fold difference between highest and lowest peak levels (Cmax: 0.11 to 2.3 micromolar after a 20 mg/m2 dose) has been reported.
Significant interindividual variability has also been noted in time to peak concentration (Tmax: 0.67 to 4 hrs after a 15 mg/m2 dose) and fraction of dose absorbed. The absorption of doses greater than 40 mg/m2 has been reported to be significantly less than that of lower doses. Food has been shown to delay absorption and reduce peak concentration. Methotrexate is generally completely absorbed from parenteral routes of injection. After intramuscular injection, peak serum concentrations occur in 30 to 60 minutes. As in leukemic pediatric patients, a wide interindividual variability in the plasma concentrations of methotrexate has been reported in pediatric patients with JIA. Following oral administration of methotrexate in doses of 6.4 to 11.2 mg/m2/week in pediatric patients with JIA, mean serum concentrations were 0.59 micromolar (range, 0.03 to 1.40) at 1 hour, 0.44 micromolar (range, 0.01 to 1.00) at 2 hours, and 0.29 micromolar (range, 0.06 to 0.58) at 3 hours.
Distribution
After intravenous administration, the initial volume of distribution is approximately 0.18 L/kg (18% of body weight) and steady-state volume of distribution is approximately 0.4 to 0.8 L/kg (40 to 80% of body weight). Methotrexate competes with reduced folates for active transport across cell membranes by means of a single carrier-mediated active transport process. At serum concentrations greater than 100 micromolar, passive diffusion becomes a major pathway by which effective intracellular concentrations can be achieved. Methotrexate in serum is approximately 50% protein bound. Laboratory studies demonstrate that it may be displaced from plasma albumin by various compounds including sulfonamides, salicylates, tetracyclines, chloramphenicol, and phenytoin.
Methotrexate does not penetrate the blood-cerebrospinal fluid barrier in therapeutic amounts when given orally or parenterally. High CSF concentrations of the drug may be attained by intrathecal administration of other parenteral forms of methotrexate.
In dogs, synovial fluid concentrations after oral dosing were higher in inflamed than uninflamed joints. Although salicylates did not interfere with this penetration, prior prednisone treatment reduced penetration into inflamed joints to the level of normal joints.
Metabolism
After absorption, methotrexate undergoes hepatic and intracellular metabolism to polyglutamated forms which can be converted back to methotrexate by hydrolase enzymes. These polyglutamates act as inhibitors of dihydrofolate reductase and thymidylate synthetase. Small amounts of methotrexate polyglutamates may remain in tissues for extended periods. The retention and prolonged drug action of these active metabolites vary among different cells, tissues and tumors. A small amount of metabolism to 7-hydroxymethotrexate may occur at doses commonly prescribed. Accumulation of this metabolite may become significant at the high doses used in osteogenic sarcoma. The aqueous solubility of 7-hydroxymethotrexate is 3 to 5 fold lower than the parent compound. Methotrexate is partially metabolized by intestinal flora after oral administration.
Half-Life
The terminal half-life reported for methotrexate is approximately three to ten hours for patients receiving treatment for psoriasis, or rheumatoid arthritis or low dose antineoplastic therapy (less than 30 mg/m2). For patients receiving high doses of methotrexate, the terminal half-life is eight to 15 hours.
In pediatric patients receiving methotrexate for acute lymphocytic leukemia (6.3 to 30 mg/m2), or for JIA (3.75 to 26.2 mg/m2), the terminal half-life has been reported to range from 0.7 to 5.8 hours or 0.9 to 2.3 hours, respectively.
Excretion
Renal excretion is the primary route of elimination and is dependent upon dosage and route of administration. With IV administration, 80% to 90% of the administered dose is excreted unchanged in the urine within 24 hours. There is limited biliary excretion amounting to 10% or less of the administered dose. Enterohepatic recirculation of methotrexate has been proposed.
Renal excretion occurs by glomerular filtration and active tubular secretion. Nonlinear elimination due to saturation of renal tubular reabsorption has been observed in psoriatic patients at doses between 7.5 and 30 mg. Impaired renal function, as well as concurrent use of drugs such as weak organic acids that also undergo tubular secretion, can markedly increase methotrexate serum levels.
Excellent correlation has been reported between methotrexate clearance and endogenous creatinine clearance. Methotrexate clearance rates vary widely and are generally decreased at higher doses. Delayed drug clearance has been identified as one of the major factors responsible for methotrexate toxicity. It has been postulated that the toxicity of methotrexate for normal tissues is more dependent upon the duration of exposure to the drug rather than the peak level achieved. When a patient has delayed drug elimination due to compromised renal function, a third space effusion, or other causes, methotrexate serum concentrations may remain elevated for prolonged periods.
When other forms of parenteral methotrexate are administered during cancer chemotherapy, the potential for toxicity from high dose regimens or delayed excretion is reduced by the administration of leucovorin calcium during the final phase of methotrexate plasma elimination.
Pharmacokinetic monitoring of methotrexate serum concentrations may help identify those patients at high risk for methotrexate toxicity and aid in proper adjustments of leucovorin dosing.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Methotrexate has been evaluated in a number of animal studies for carcinogenic potential with inconclusive results. Although there is evidence that methotrexate causes chromosomal damage to animal somatic cells and human bone marrow cells, the clinical significance remains uncertain.
Data are available regarding the risks for pregnancy and for fertility in humans [see Use in Specific Populations (8.1 and 8.3)].
-
14 CLINICAL STUDIES
14.1 Rheumatoid Arthritis
Clinical trials in patients with rheumatoid arthritis were performed using other formulations of methotrexate.
In patients with rheumatoid arthritis, effects of methotrexate on articular swelling and tenderness can be seen as early as 3 to 6 weeks.
Most studies of methotrexate in patients with rheumatoid arthritis are relatively short term (3 to 6 months). Limited data from long-term studies indicate that an initial clinical improvement is maintained for at least two years with continued therapy.
14.2 Polyarticular Juvenile Idiopathic Arthritis
Clinical trials in patients with polyarticular juvenile idiopathic arthritis were performed using other formulations of methotrexate.
In a 6-month double-blind, placebo-controlled trial of 127 pediatric patients with pJIA (mean age, 10.1 years; age range, 2.5 to 18 years; mean duration of disease, 5.1 years) on background nonsteroidal anti-inflammatory drugs and/or prednisone, methotrexate given weekly at an oral dose of 10 mg/m2 provided significant clinical improvement compared to placebo as measured by either the physician’s global assessment, or by a patient composite (25% reduction in the articular-severity score plus improvement in parent and physician global assessments of disease activity). Over two-thirds of the patients in this trial had polyarticular-course JIA, and the numerically greatest response was seen in this subgroup treated with 10 mg/m2/wk methotrexate.
The overwhelming majority of the remaining patients had systemic-course JIA. All patients were unresponsive to NSAIDs; approximately one-third were using low dose corticosteroids.
Weekly methotrexate at a dose of 5 mg/m2 was not significantly more effective than placebo in this trial.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
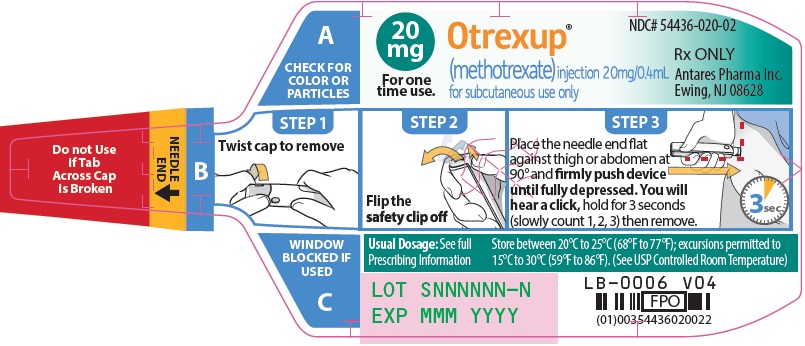
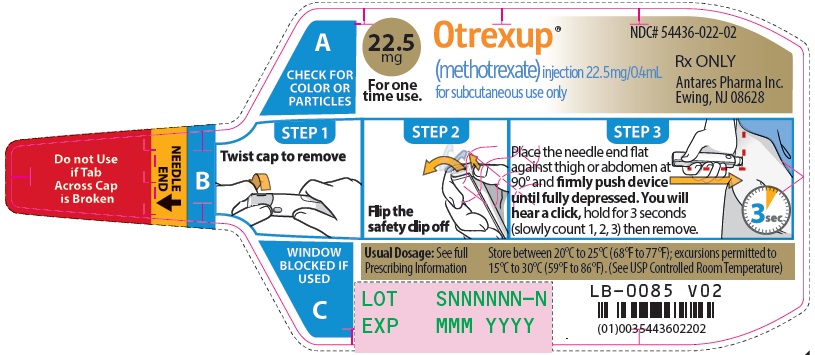
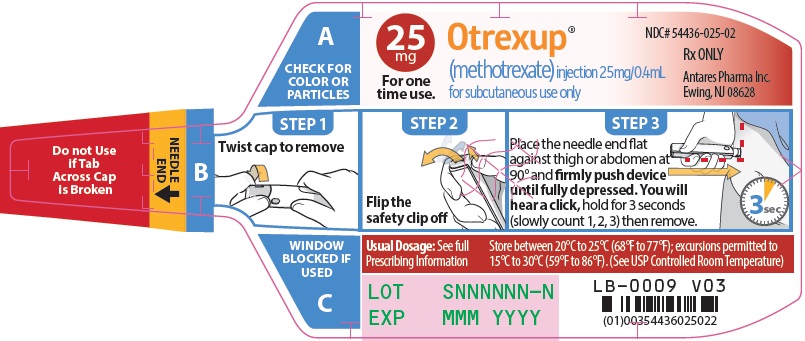
Otrexup contains methotrexate in a preservative-free sterile solution for a single subcutaneous injection. Otrexup is available in the following strengths and configurations.
Otrexup (methotrexate) injection 10 mg/0.4 mL
- Carton of 1 NDC 54436-010-01
- Carton of 4 NDC 54436-010-04
- Otrexup NDC 54436-010-02
Otrexup (methotrexate) injection 12.5 mg/0.4 mL
- Carton of 1 NDC 54436-012-01
- Carton of 4 NDC 54436-012-04
- Otrexup NDC 54436-012-02
Otrexup (methotrexate) injection 15 mg/0.4 mL
- Carton of 1 NDC 54436-015-01
- Carton of 4 NDC 54436-015-04
- Otrexup NDC 54436-015-02
Otrexup (methotrexate) injection 17.5 mg/0.4 mL
- Carton of 1 NDC 54436-017-01
- Carton of 4 NDC 54436-017-04
- Otrexup NDC 54436-017-02
Otrexup (methotrexate) injection 20 mg/0.4 mL
- Carton of 1 NDC 54436-020-01
- Carton of 4 NDC 54436-020-04
- Otrexup NDC 54436-020-02
Otrexup (methotrexate) injection 22.5 mg/0.4 mL
- Carton of 1 NDC 54436-022-01
- Carton of 4 NDC 54436-022-04
- Otrexup NDC 54436-022-02
Otrexup (methotrexate) injection 25 mg/0.4 mL
- Carton of 1 NDC 54436-025-01
- Carton of 4 NDC 54436-025-04
- Otrexup NDC 54436-025-02
Store at controlled room temperature, 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). PROTECT FROM LIGHT.
Handling and Disposal
Handle and dispose of Otrexup consistent with recommendations for handling and disposal of cytotoxic drugs.1
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information and Instructions for Use)
Risk of Organ Toxicity
Inform patients of the risks of organ toxicity, including gastrointestinal, hematologic, hepatic, infections, neurologic, pulmonary, renal and skin as well as possible signs and symptoms for which they should contact their healthcare provider. Advise patients of the need for close follow-up, including periodic laboratory tests to monitor toxicity [see Warnings and Precautions (5.1 and 5.4)].
Importance of Proper Dosing and Administration
Both the physician and pharmacist should emphasize to the patient that the recommended dose is taken weekly and that mistaken daily use of the recommended dose has led to fatal toxicity [see Dosing and Administration (2)].
Otrexup is intended for use under the guidance and supervision of a physician. Patients should not self-administer until they receive training from a healthcare professional. The patient’s or caregiver’s ability to administer Otrexup should be assessed. A trainer device is available for training purposes.
Patients should be instructed to use administration sites on the abdomen or the thigh. Administration should not be made within 2 inches of the navel. Instruct patients not to administer Otrexup to the arms or any other areas of the body, as delineated in the Otrexup Instructions for Use [see Instructions for Use].
Embryo-Fetal Toxicity
- Advise females of reproductive potential that Otrexup can cause fetal harm and is contraindicated in pregnancy. Advise women of childbearing potential that Otrexup should not be started until pregnancy is excluded. Women should be fully counseled on the serious risk to the fetus should they become pregnant while undergoing treatment. Inform patients to contact their physician if they suspect that they are pregnant [see Boxed Warning, Contraindications (4), Warnings and Precautions (5.2), Use in Specific Populations (8.1)].
- Advise females of reproductive potential to use effective contraception during Otrexup therapy and for 6 months after the final dose [see Use in Specific Populations (8.3)].
- Advise males of reproductive potential to use effective contraception during Otrexup therapy and for 3 months after the final dose [see Use in Specific Populations (8.3)].
Infertility
Advise patients of reproductive potential that Otrexup may cause impairment of fertility, oligospermia, and menstrual dysfunction [see Warnings and Precautions (5.2), Use in Specific Populations (8.3)].
Lactation
Advise females not to breastfeed during treatment with Otrexup and for one week after the final dose [see Use in Specific Populations (8.3)].
Ability to Drive or Operate Machinery
Inform patients that adverse reactions such as dizziness and fatigue may affect their ability to drive or operate machinery.
Proper Storage and Disposal
Advise patients to store Otrexup at room temperature (68 to 77°F or 20 to 25°C). Inform patients and caregivers of the need for proper disposal after use, including the use of a sharps disposal container.
Address Medical Inquiries to:
Antares Pharma, Inc.
Medical Communications
100 Princeton South, Suite 300
Ewing, NJ 08628
1-855-Otrexup (1-855-687-3987)Manufactured for:
Antares Pharma, Inc.
100 Princeton South, Suite 300
Ewing, NJ 08628 USA
Otrexup™ is the subject of US Patent Nos. 8,021,335, 6,746,429, 8,480,631, 8,562,564, 8,579,865, and 8,945,063.©2019 Antares Pharma, Inc., Ewing, NJ 08628

-
Patient Package Insert
PATIENT INFORMATION
Otrexup™ (oh-TREKS-up)
(methotrexate)
injection, for subcutaneous use
What is Otrexup?
Otrexup is a single-dose auto-injector containing a prescription medicine, methotrexate.
Methotrexate is used to:- treat certain adults with severe, active rheumatoid arthritis (RA), and children with active polyarticular juvenile idiopathic arthritis (pJIA), after treatment with other medicines including non-steroidal anti-inflammatory (NSAIDS) have been used and did not work well.
- control the symptoms of severe, resistant, disabling psoriasis in adults when other types of treatment have been used and did not work well.
Otrexup is available in doses of 10, 12.5, 15, 17.5, 20, 22.5 and 25 mg. Your doctor will prescribe a different way to take methotrexate if you need to take methotrexate by mouth or in some other way. Your doctor may also change your prescription if your dose does not match the available Otrexup doses, such as doses of less than 10 mg, more than 25 mg, or doses in between the available Otrexup doses.
Otrexup should not be used for the treatment of cancer.
Otrexup should not be used for the treatment of children with psoriasis.
What is the most important information I should know about Otrexup?
Otrexup can cause serious side effects that can lead to death, including:
1. Organ system toxicity. People who use methotrexate for the treatment of cancer, psoriasis, or rheumatoid arthritis, have an increased risk of death from organ toxicity. Types of organ toxicity can include:
o gastrointestinal
o bone marrow
o liver
o immune system
o nerve
o lung
o kidneys
o skin
Your doctor will do blood tests and other types of tests before you take and while you are taking Otrexup to check for signs and symptoms of organ toxicity. Call your doctor right away if you have any of the following symptoms of organ toxicity:
o vomiting
o diarrhea
o mouth sores
o fever
o confusion
o weakness
o temporary blindness
o seizures
o headache
o back pain
o neck stiffness
o paralysis
o irritability
o sleepiness
o problems with coordination
o dry cough
o trouble breathing
o severe skin rash
2. Women who are pregnant are at increased risk for death of the baby and birth defects. Women who are pregnant or who plan to become pregnant must not take Otrexup. A pregnancy test should be performed before starting Otrexup.
Contraception should be used by both females and males while taking Otrexup. Pregnancy should be avoided if either partner is receiving Otrexup:
- for a minimum of 3 months after treatment with Otrexup for males.
- during and for 6 months after treatment with Otrexup for females.
Who should not take Otrexup?
Do not take Otrexup if you:
- are pregnant or planning to become pregnant. See “What is the most important information I should know about Otrexup?"
- are breastfeeding.
- Otrexup can pass into your breast milk and may harm your baby. Do not breastfeed while taking Otrexup. Talk to your doctor about the best way to feed your baby if you take Otrexup.
- have alcohol problems (alcoholism).
- have liver problems.
- have problems fighting infection (immunodeficiency syndrome).
- have been told you have (or think you have) a blood disorder such as low levels of white blood cells, red blood cells (anemia), or platelets.
- have had an allergy to methotrexate or any of the ingredients in Otrexup. See the end of this leaflet for a complete list of ingredients in Otrexup.
Talk to your doctor before taking this medicine if you have any of these conditions.
What should I tell my doctor before taking Otrexup?
Before you take Otrexup, tell your doctor if you have any other medical conditions.
Tell your doctor about all of the medicines you take, including prescription, over-the-counter medicines, vitamins, and herbal supplements.
Otrexup may affect how other medicines work, and other medicines may affect how Otrexup works causing side effects.
Ask your doctor or pharmacist for a list of medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take Otrexup?
- Read the Instructions for Use that come with Otrexup.
- Take Otrexup exactly as your doctor tells you to take it.
- Inject Otrexup only 1 time each week. Do not take Otrexup every day.
Taking Otrexup every day may cause death from toxicity. - Your doctor will show you or your caregiver how to inject Otrexup. You should not inject Otrexup until you have been trained on the right way to use it.
- Check Otrexup before you inject it. Otrexup should be yellow in color and should not have any lumps or particles in it.
- Otrexup should be injected in the stomach (abdomen) or thigh.
- Do not inject Otrexup within 2 inches of the belly button (navel).
- Do not inject Otrexup in the arms or any other areas of the body.
- Do not inject Otrexup in areas where the skin is tender, bruised, red, scaly, hard, or has scars or stretch marks.
- If you are not sure if Otrexup was injected, or if you have hard time giving the injection, do not inject another dose. Call your pharmacist or doctor right away.
- If you inject too much Otrexup, call your doctor or go to the nearest hospital emergency room right away.
What should I avoid while taking Otrexup?
- Do not drink alcohol while taking Otrexup. Drinking alcohol can increase your chances of getting serious side effects.
- Otrexup can cause dizziness and tiredness. Do not drive a car, operate machinery, or do anything that needs you to be alert until you know how Otrexup affects you.
- Certain vaccinations should be avoided while taking Otrexup. Talk to your doctor before you or members of your household receive any vaccines.
What are the possible side effects of Otrexup?
Otrexup may cause serious side effects, including:
See “What is the most important information I should know about Otrexup?"
- fertility problems. Methotrexate, the active ingredient in Otrexup, may affect your ability to have a baby. Males may have a decreased sperm count, and females may have changes to their menstrual cycle. This can happen while taking Otrexup and for a short period of time after you stop.
- certain cancers. Some people who have taken methotrexate have had a certain type of cancer called Non-Hodgkin’s lymphoma and other tumors. Your doctor may tell you to stop taking Otrexup if this happens.
- tissue and bone problems. Taking Methotrexate while having radiation therapy may increase the risk of your tissue or bone not receiving enough blood. This may lead to death of the tissue or bone.
Common side effects of Otrexup include:
• nausea
• stomach pain
• indigestion (dyspepsia)
• mouth sores
• rash
• stuffy or runny nose and sore throat
• diarrhea
• abnormal liver function tests
• vomiting
• headache
• bronchitis
• low red, white, and platelet blood cell count
• hair loss
• dizziness
• sensitivity to light
• burning skin lesions
• lung problems
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Otrexup. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I dispose of Otrexup?
- Do not throw away in the household trash. Put used Otrexup in a FDA-cleared sharps disposal container right away after use.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Safely dispose of Otrexup that is out of date or is no longer needed.
How should I store Otrexup?
Store Otrexup at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze
- Keep Otrexup out of the light.
Keep Otrexup and all medicines out of the reach of children.
General information about the safe and effective use of Otrexup.
Methotrexate is sometimes prescribed for purposes other than those listed in Patient Information leaflet. Do not use Otrexup for a condition for which it was not prescribed. Do not give Otrexup to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Otrexup. If you would like more information, talk to your doctor. You can ask your doctor or pharmacist for information about Otrexup that is written for health professionals.
For more information, go to www.Otrexup.com or call 1-855-Otrexup (1-855-687-3987).
What are the ingredients in Otrexup?
Active ingredient: methotrexate
Inactive ingredients: hydrochloric acid, sodium chloride, sodium hydroxide and water for injection, USP.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Antares Pharma, Inc.
100 Princeton South, Suite 300
Ewing, NJ 08628 USA
©2019 Antares Pharma, Inc., Ewing, NJ 08628
Issued: 12/2019
-
Instructions for Use
Read this Instructions for Use before using Otrexup™ (oh-TREKS-up) (methotrexate) injection, for subcutaneous use
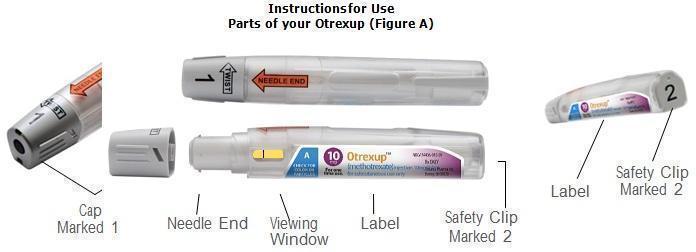
Prepare to Use Otrexup
- Do not remove cap (marked 1) or safety clip (marked 2) until you are ready to inject Otrexup.
- Wash your hands well with soap and warm water.
- Check the expiration date on the label. Do not use if expired.
- Check the seal. Do not use Otrexup if the seal is broken (See Figure B).
- In addition to Otrexup, you will need the following items: 1 alcohol swab and 1 cotton ball or gauze.
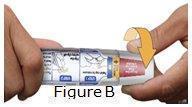
Check the Liquid
- Look at the viewing window (See Figure C).
- The liquid should be yellow in color and should not have any lumps or particles in it.
- You may see an air bubble. This is normal.
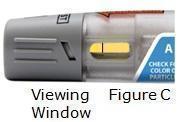
Choose an Injection Site
- Otrexup should be injected into the stomach (abdomen) or thigh. Do not inject Otrexup within 2 inches of the belly button (navel) (See Figure D).
- Do not inject Otrexup in the arms or any other areas of the body.
- Do not inject Otrexup in areas where the skin is tender, bruised, red, scaly, hard, or has scars or stretch marks.
- Wipe the area with an alcohol swab.
- Allow the skin to dry and do not touch this area again before giving Otrexup. Do not fan or blow on the clean area.
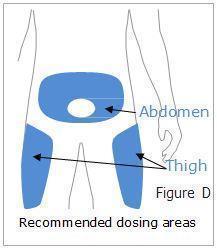
Give your Injection
Step 1: Remove Cap (marked 1)
- Twist cap marked 1 to remove (See Figure E). This will break the seal.
- You may notice 1 or 2 drops of medicine. This is normal.
- Do not touch the needle end with your hands or fingers. This could inject the medicine into your hand.
- Do not replace the cap after it has been removed.
- After the cap is removed Otrexup must be used or disposed of safely (See step 5).
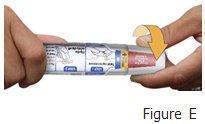
STEP 2: Remove the Safety Clip (marked 2)
- Flip the Safety Clip marked 2 (See Figure F).
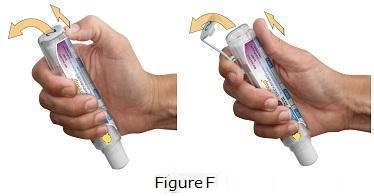
STEP 3: Inject Otrexup
- Place needle end of Otrexup flat against thigh or stomach (abdomen) at a 90° and firmly push device against the injection site until fully depressed. You will hear a click, hold for 3 seconds (slowly count 1,2,3) (See Figure G).
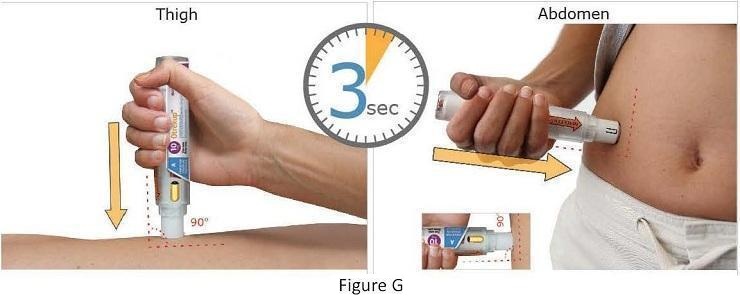
- After counting to 3, remove Otrexup from the injection site. You may notice a small amount of blood or liquid at the administration site, which is normal.
- Press a cotton ball or gauze on the area for 10 seconds. Do not rub the area.
Step 4: Check the Viewing Window
- Check the viewing window (See Figure H).
- The viewing window will be half blocked with a red flag to show that the full dose was delivered. (See Figure H).
- Dispose of Otrexup in a puncture-proof disposable container (See Step 5).
- If the viewing window is not half blocked with a red flag, call your doctor or pharmacist, or call 1-855-Otrexup (1-855-687-3987) for help.
- Do not use another Otrexup without talking to your doctor.
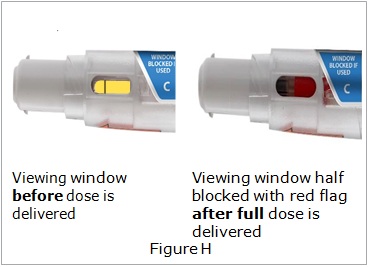
Step 5:
Dispose of Otrexup
- Do not throw away in the household trash. Put used Otrexup in a FDA-cleared sharps disposal container right away after use.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Safely dispose of Otrexup that is out of date or no longer needed.
How should I store Otrexup?
- Store Otrexup at room temperature between 68°F to 77°F (20°C to 25°C). Do not freeze.
- Keep Otrexup out of the light.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Mfd. For Antares Pharma, Inc. Ewing, NJ 08628
12/2019
PII-13-001 V11
- PRINCIPAL DISPLAY PANEL - Autoinjector
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL - Carton of 4
-
INGREDIENTS AND APPEARANCE
OTREXUP
methotrexate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54436-075 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOTREXATE (UNII: YL5FZ2Y5U1) (METHOTREXATE - UNII:YL5FZ2Y5U1) METHOTREXATE 7.5 mg in 0.4 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54436-075-01 1 in 1 CARTON 10/11/2013 09/30/2016 1 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:54436-075-04 4 in 1 CARTON 10/11/2013 09/30/2016 2 NDC:54436-075-02 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204824 10/11/2013 09/30/2016 OTREXUP
methotrexate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54436-010 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOTREXATE (UNII: YL5FZ2Y5U1) (METHOTREXATE - UNII:YL5FZ2Y5U1) METHOTREXATE 10 mg in 0.4 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54436-010-01 1 in 1 CARTON 10/11/2013 1 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:54436-010-03 1 in 1 CARTON 10/11/2013 2 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:54436-010-04 4 in 1 CARTON 10/11/2013 3 NDC:54436-010-02 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204824 10/11/2013 OTREXUP
methotrexate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54436-012 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOTREXATE (UNII: YL5FZ2Y5U1) (METHOTREXATE - UNII:YL5FZ2Y5U1) METHOTREXATE 12.5 mg in 0.4 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54436-012-01 1 in 1 CARTON 03/24/2016 1 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:54436-012-03 1 in 1 CARTON 03/24/2016 2 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:54436-012-04 4 in 1 CARTON 03/24/2016 3 NDC:54436-012-02 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204824 10/11/2013 OTREXUP
methotrexate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54436-015 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOTREXATE (UNII: YL5FZ2Y5U1) (METHOTREXATE - UNII:YL5FZ2Y5U1) METHOTREXATE 15 mg in 0.4 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54436-015-01 1 in 1 CARTON 10/11/2013 1 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:54436-015-03 1 in 1 CARTON 10/11/2013 2 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:54436-015-04 4 in 1 CARTON 10/11/2013 3 NDC:54436-015-02 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204824 10/11/2013 OTREXUP
methotrexate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54436-017 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOTREXATE (UNII: YL5FZ2Y5U1) (METHOTREXATE - UNII:YL5FZ2Y5U1) METHOTREXATE 17.5 mg in 0.4 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54436-017-01 1 in 1 CARTON 03/24/2016 1 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:54436-017-03 1 in 1 CARTON 03/24/2016 2 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:54436-017-04 4 in 1 CARTON 03/24/2016 3 NDC:54436-017-02 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204824 10/11/2013 OTREXUP
methotrexate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54436-020 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOTREXATE (UNII: YL5FZ2Y5U1) (METHOTREXATE - UNII:YL5FZ2Y5U1) METHOTREXATE 20 mg in 0.4 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54436-020-01 1 in 1 CARTON 10/11/2013 1 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:54436-020-03 1 in 1 CARTON 10/11/2013 2 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:54436-020-04 4 in 1 CARTON 10/11/2013 3 NDC:54436-020-02 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204824 10/11/2013 OTREXUP
methotrexate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54436-022 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOTREXATE (UNII: YL5FZ2Y5U1) (METHOTREXATE - UNII:YL5FZ2Y5U1) METHOTREXATE 22.5 mg in 0.4 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54436-022-01 1 in 1 CARTON 03/24/2016 1 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:54436-022-03 1 in 1 CARTON 03/24/2016 2 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:54436-022-04 4 in 1 CARTON 03/24/2016 3 NDC:54436-022-02 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204824 10/11/2013 OTREXUP
methotrexate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54436-025 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOTREXATE (UNII: YL5FZ2Y5U1) (METHOTREXATE - UNII:YL5FZ2Y5U1) METHOTREXATE 25 mg in 0.4 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54436-025-01 1 in 1 CARTON 10/11/2013 1 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:54436-025-03 1 in 1 CARTON 10/11/2013 2 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:54436-025-04 4 in 1 CARTON 10/11/2013 3 NDC:54436-025-02 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204824 10/11/2013 Labeler - Antares Pharma, Inc. (085369585) Establishment Name Address ID/FEI Business Operations Pharmascience Inc. 202657094 manufacture(54436-010, 54436-012, 54436-015, 54436-017, 54436-020, 54436-022, 54436-025) Establishment Name Address ID/FEI Business Operations Sharp Packaging Services, LLC 143696495 PACK(54436-010, 54436-012, 54436-015, 54436-017, 54436-020, 54436-022, 54436-025) Establishment Name Address ID/FEI Business Operations NeoPharm Labs Inc 243379372 ANALYSIS(54436-010, 54436-012, 54436-015, 54436-017, 54436-020, 54436-022, 54436-025) Establishment Name Address ID/FEI Business Operations PPD Development, L.P. 838082055 analysis(54436-010, 54436-012, 54436-015, 54436-017, 54436-020, 54436-022, 54436-025)