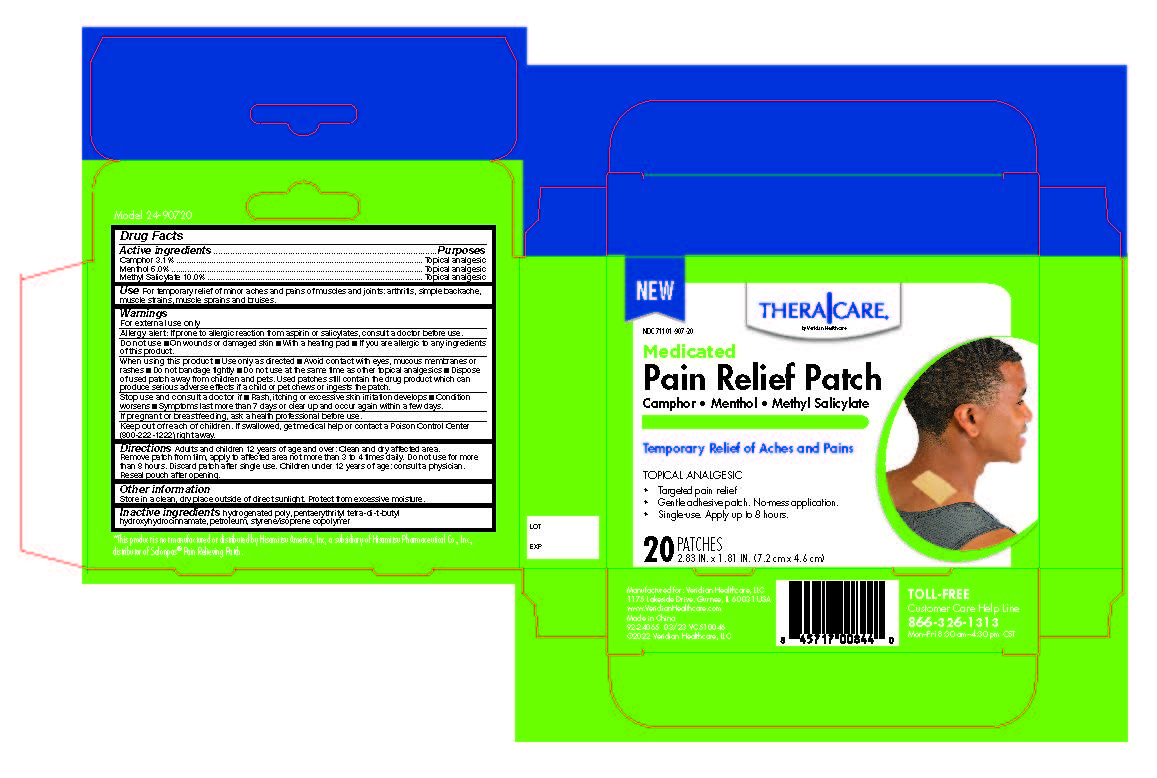
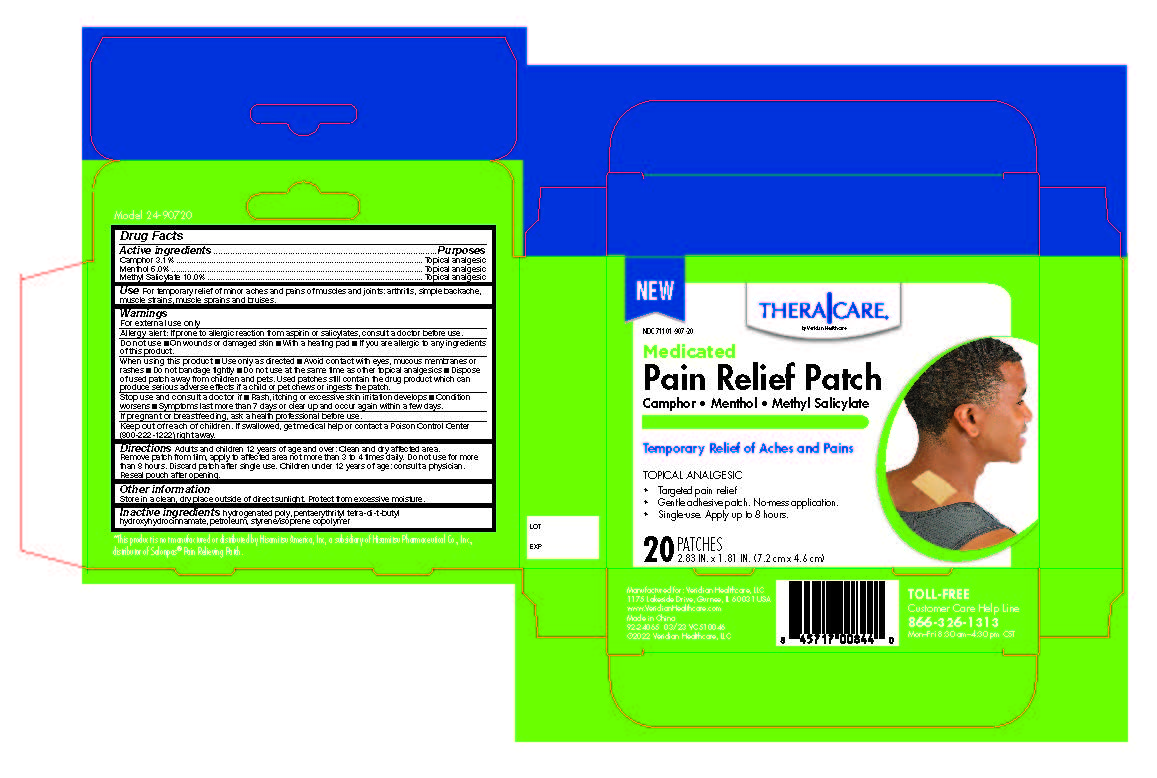
Label: PAIN RELIEF PATCHES- camphor, menthol, methyl salicylate patch
- NDC Code(s): 71101-907-20
- Packager: Veridian Healthcare
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active Ingredients
Active Ingredients Purpose - Camphor 3.1% ......................Topical Analgesic - Menthol 6.0% .......................Topical Analgesic - Methyl Salicylate 10.0% .......Topical ...
-
For External Use Only
Allergy alert: If prone to allergic reaction from asprin or salicylates, consult a doctor before use
-
Do not use:On wounds or damaged skin - With a heating pad - If you are allergic to any ingredients of this product
-
When using this product
Use only as directed - Avoid contact with eyes, mucous membranes or rashes - Do not bandage tighly - Do not use at the same time as other topical analgesics - Dispose of used patch in manner that keeps ...
-
Stop use and consult a doctor
If rash, itching or excessive skin irritation develops - If condition worsens - if symptoms last more than 7 days or clear up and occur again with a few days
-
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
-
Uses
For temporary relief of minor aches and pains of muscles and joints associated with arthritis, simple backache, strains, sprains, and bruises.
-
Directions
Adults and children 12 years of age and over: Clean and dry affected area. Remove patch from film, apply to affected area not more than 3 to 4 times daily. Remove patch from the skin after at ...
-
Pain Relief Patch LabelOther Information: Store in clean, dy place outside of direct sunlight. Protect from excessive moisture.
-
INGREDIENTS AND APPEARANCEProduct Information