Label: PROPARACAINE HYDROCHLORIDE solution/ drops
- NDC Code(s): 68071-5139-5
- Packager: NuCare Pharmaceuticals,Inc.
- This is a repackaged label.
- Source NDC Code(s): 0998-0016
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 2, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
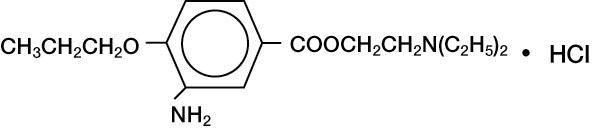
DESCRIPTION:ALCAINE - ® (proparacaine hydrochloride ophthalmic solution, USP) 0.5% is a topical local anesthetic for ophthalmic use. The active ingredient is represented by the structural formula ...
-
CLINICAL PHARMACOLOGY:ALCAINE - ® ophthalmic solution is a rapidly-acting topical anesthetic, with induced anesthesia lasting approximately 10-20 minutes.
-
INDICATIONS AND USAGE:ALCAINE - ® ophthalmic solution is indicated for procedures in which a topical ophthalmic anesthetic is indicated: corneal anesthesia of short duration, e.g. tonometry, gonioscopy ...
-
CONTRAINDICATIONS:ALCAINE - ® ophthalmic solution should be considered contraindicated in patients with known hypersensitivity to any of the ingredients of this preparation.
-
WARNINGS:NOT FOR INJECTION - FOR TOPICAL OPHTHALMIC USE ONLY. Prolonged use of a topical ocular anesthetic is not recommended. It may produce permanent corneal opacification with accompanying visual ...
-
PRECAUTIONS:Carcinogenesis, Mutagenesis, Impairment of Fertility. Long-term studies in animals have not been performed to evaluate carcinogenic potential, mutagenicity, or possible impairment of fertility ...
-
Nursing Mothers:It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when proparacaine hydrochloride is administered to a nursing ...
-
Pediatric Use:Safety and effectiveness of proparacaine hydrochloride ophthalmic solution in pediatric patients have been established. Use of proparacaine hydrochloride is supported by evidence from adequate and ...
-
Geriatric Use:No overall clinical differences in safety or effectiveness have been observed between the elderly and other adult patients.
-
ADVERSE REACTIONS:Occasional temporary stinging, burning and conjunctival redness may occur with the use of proparacaine. A rare, severe, immediate-type, apparently hyperallergic corneal reaction characterized by ...
-
DOSAGE AND ADMINISTRATION:Usual Dosage: Removal of foreign bodies and sutures, and for tonometry: 1 to 2 drops (in single instillations) in each eye before operating. Short corneal and conjunctival procedures: 1 drop in ...
-
HOW SUPPLIED:ALCAINE - ® (proparacaine hydrochloride ophthalmic solution, USP) 0.5% is supplied in 15 mL DROP-TAINER - ® dispensers. NDC 68071-5139-5 - Storage: Bottle must be stored in unit ...
-
PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information