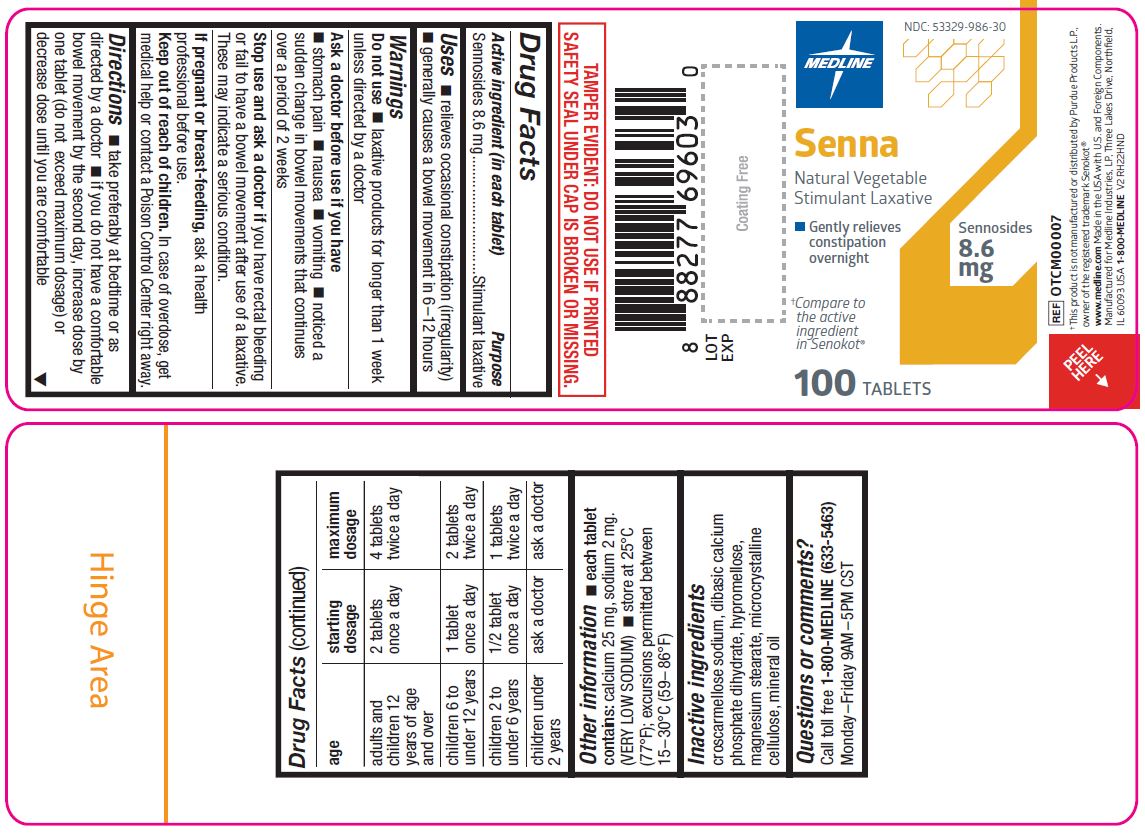
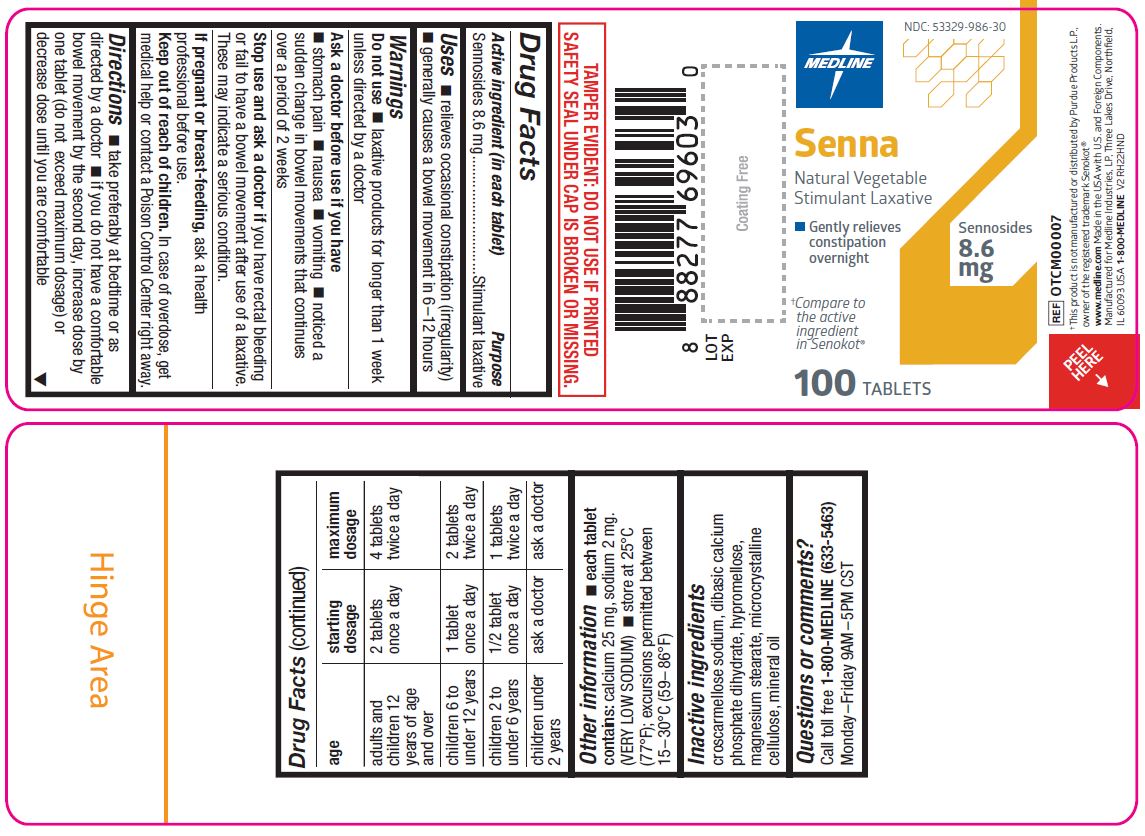
Label: SENNA NATURAL VEGETABLE- sennosides tablet, coated
- NDC Code(s): 53329-986-30
- Packager: Medline Industries, LP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
- Warnings
-
Directions
- take preferably at bedtime or as directed by a doctor
- if you do not have a comfortable bowel movement by the second day, increase dose by one tablet (do not exceed maximum dosage) or decrease dose until you are comfortable
age starting dosage maximum dosage adults and children 12 years age and over 2 tablets once a day 4 tablets twice a day children 6 to under 12 years 1 tablet once a day 2 tablets twice a day children 2 to under 6 years 1/2 tablet once a day 1 tablet twice a day children under 2 years ask a doctor ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
- Manufacturing Information
- Package Label
-
INGREDIENTS AND APPEARANCE
SENNA NATURAL VEGETABLE
sennosides tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53329-986 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES A AND B (UNII: 1B5FPI42EN) (SENNOSIDES A AND B - UNII:1B5FPI42EN) SENNOSIDES A AND B 8.6 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MINERAL OIL (UNII: T5L8T28FGP) Product Characteristics Color brown Score score with uneven pieces Shape ROUND Size 9mm Flavor Imprint Code TCL080 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53329-986-30 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 01/01/2019 Labeler - Medline Industries, LP (025460908)