Label: ELCYS- cysteine hydrochloride injection, solution
- NDC Code(s): 51754-1008-4
- Packager: Exela Pharma Sciences, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ELCYS® safely and effectively. See full prescribing information for ELCYS®. ELCYS® (cysteine hydrochloride injection), for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE ELCYS® is indicated for use as an additive to amino acid solutions to meet the nutritional requirements of newborn infants requiring total parenteral nutrition (TPN); and of adult and pediatric ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Important Administration Information - Prior to administration, ELCYS® must be diluted and used as an admixture in PN solutions. ELCYS® is not for direct intravenous infusion. The resulting ...
-
3 DOSAGE FORMS AND STRENGTHS Injection - • Pharmacy Bulk Package: 2500 mg/50mL (50 mg/mL) cysteine hydrochloride, USP in a clear, colorless, sterile solution in a 50 mL vial.
-
4 CONTRAINDICATIONS ELCYS® is contraindicated in: • Patients with known hypersensitivity to one or more amino acids. • Patients with inborn errors of amino acid metabolism due to risk of severe metabolic or ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Pulmonary Embolism due to Pulmonary Vascular Precipitates - Pulmonary vascular precipitates causing pulmonary vascular emboli and pulmonary distress have been reported in patients receiving ...
-
6 ADVERSE REACTIONS The following serious adverse reactions are discussed in greater detail in other sections of the prescribing information: • Pulmonary embolism due to pulmonary vascular precipitates [see Warnings ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Appropriate administration of ELCYS® is not expected to cause major birth defects, miscarriage or adverse maternal or fetal outcomes. Animal reproduction studies ...
-
10 OVERDOSAGE In the event of over hydration or solute overload, re-evaluate the patient and institute appropriate corrective measures [see Warnings and Precautions (5.3, 5.4, 5.5, 5.7, 5.8)].
-
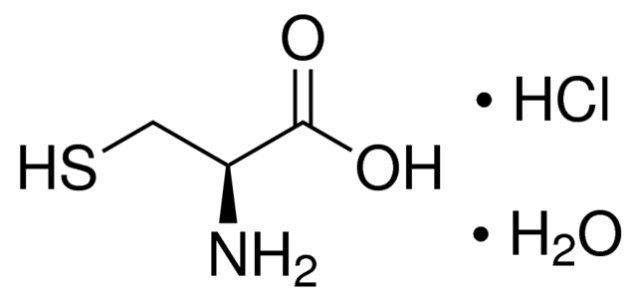
11 DESCRIPTION ELCYS® (cysteine hydrochloride injection) is a sterile, nonpyrogenic solution for intravenous use. Each 50 mL of ELCYS® contains 2500 mg of cysteine hydrochloride, USP (equivalent to 345 mg of ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Endogenous cysteine is synthesized from methionine by the enzyme, cystathionase, via the trans-sulfuration pathway, and serves as a precursor substrate for both ...
-
15 REFERENCES 1. Ayers P. et al. A.S.P.E.N. Parenteral Nutrition Handbook, 2nd ed. 2014 pg. 123 and 124.
-
16 HOW SUPPLIED/STORAGE AND HANDLING ELCYS® is supplied as follows: Pharmacy Bulk Package: 2,500 mg/50 mL (50 mg/mL) of cysteine hydrochloride, USP is a clear, colorless, sterile and nonpyrogenic solution in 50 mL vials packaged as 1 ...
-
17 PATIENT COUNSELING INFORMATION Inform patients, caregivers, or home healthcare providers of the following risks of ELCYS®: • Pulmonary embolism due to pulmonary vascular precipitates [see Warnings and Precautions ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-Vial Label Rx Only NDC 51754-1008-1 - ELCYS® (cysteine hydrochloride injection), USP - 2,500 mg/50 mL (50 mg/mL) Pharmacy Bulk Package - Not for direct infusion. Must be diluted. For intravenous ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-Carton Label Rx Only NDC 51754-1008-4 - ELCYS® (cysteine hydrochloride injection), USP - 2,500 mg/50 mL (50 mg/mL) Pharmacy Bulk Package - Not for direct infusion. Must be diluted. For intravenous ...
-
INGREDIENTS AND APPEARANCEProduct Information