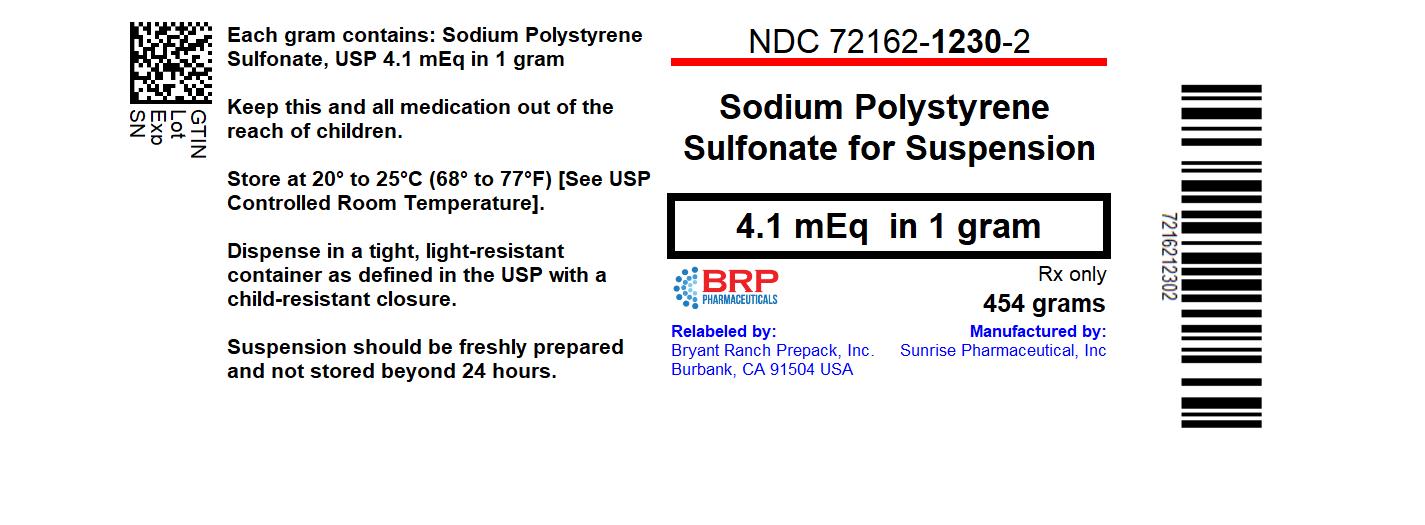
Label: SODIUM POLYSTYRENE SULFONATE powder, for suspension
- NDC Code(s): 72162-1230-2, 72162-1230-7
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 11534-166
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 12, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use sodium polystyrene sulfonate for suspension safely and effectively. See full prescribing information for sodium polystyrene sulfonate ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESodium polystyrene sulfonate for suspension is indicated for the treatment of hyperkalemia. Limitation of Use: Sodium polystyrene sulfonate for suspension should not be used as an emergency ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Information - Administer sodium polystyrene sulfonate at least 3 hours before or 3 hours after other oral medications. Patients with gastroparesis may require a 6 hour separation ...
-
3 DOSAGE FORMS AND STRENGTHSSodium polystyrene sulfonate for suspension is a golden brown, finely ground powder and is available in 454 g jars and 15 g bottles.
-
4 CONTRAINDICATIONSSodium polystyrene sulfonate is contraindicated in patients with the following conditions: Hypersensitivity to polystyrene sulfonate resins - Obstructive bowel disease - Neonates with reduced gut ...
-
5 WARNINGS AND PRECAUTIONS5.1 Intestinal Necrosis - Cases of intestinal necrosis, some fatal, and other serious gastrointestinal adverse events (bleeding, ischemic colitis, perforation) have been reported in association ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed elsewhere in the labeling: Intestinal Necrosis [see - Warnings and Precautions (5.1)] Electrolyte Disturbances [see - Warnings and ...
-
7 DRUG INTERACTIONS7.1 General Interactions - No formal drug interaction studies have been conducted in humans. Sodium polystyrene sulfonate has the potential to bind other drugs. In - in vitrobinding studies ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Sodium polystyrene sulfonate is not absorbed systemically following oral or rectal administration and maternal use is not expected to result in fetal risk ...
-
10 OVERDOSAGEOverdosage may result in electrolyte disturbances including hypokalemia, hypocalcemia, and hypomagnesemia. Appropriate measures should be taken to correct serum electrolytes (potassium, calcium ...
-
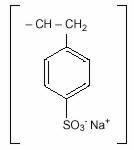
11 DESCRIPTIONSodium polystyrene sulfonate is a benzene, diethenyl- polymer, with ethenylbenzene, sulfonated, sodium salt and has the following structural formula: n - The drug is a golden brown ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sodium polystyrene sulfonate is a non-absorbed, cation exchange polymer that contains a sodium counterion. Sodium polystyrene sulfonate increases fecal potassium ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Studies have not been performed.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSodium polystyrene sulfonate for suspension is available as a golden brown, finely ground powder. NDC: 72162-1230-02: in jars of 1 pound (454 g). NDC: 72162-1230-07: in bottles of 15 g, Store at ...
-
17 PATIENT COUNSELING INFORMATIONDrug Interactions - Advise patients who are taking other oral medication to separate the dosing of sodium polystyrene sulfonate by at least 3 hours (before or after) [ see - Dosage and ...
-
PRINCIPAL DISPLAY PANELSodium Polystyrene Sulfonate Powder
-
INGREDIENTS AND APPEARANCEProduct Information

 n
n