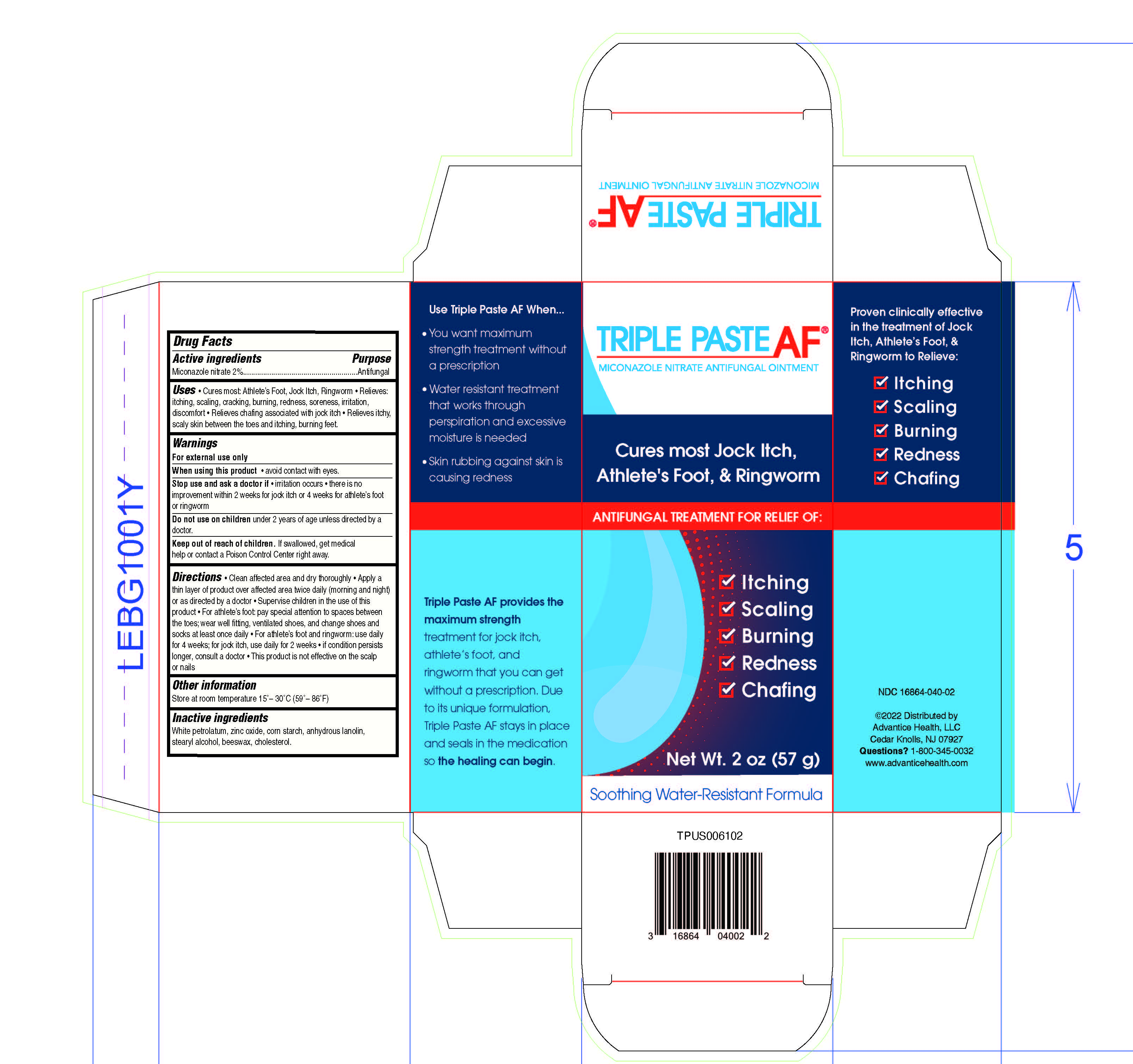
Label: TRIPLE ANTIFUNGAL- miconazole nitrate ointment
- NDC Code(s): 16864-040-02
- Packager: Advantice Health, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- Clean affected area and dry thoroughly
- Apply a thin layer of product over affected area twice daily (morning and night) or as directed by a doctor
- Supervise children in the use of this product
- Use daily for 2 weeks; If condition persists longer, consult a doctor
- This product is not effective on the scalp or nails
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 56.7 g Tube Carton
-
INGREDIENTS AND APPEARANCE
TRIPLE ANTIFUNGAL
miconazole nitrate ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:16864-040 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MICONAZOLE NITRATE (UNII: VW4H1CYW1K) (MICONAZOLE - UNII:7NNO0D7S5M) MICONAZOLE NITRATE 2 g in 100 g Inactive Ingredients Ingredient Name Strength WHITE PETROLATUM (UNII: B6E5W8RQJ4) STARCH, CORN (UNII: O8232NY3SJ) LANOLIN (UNII: 7EV65EAW6H) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) YELLOW WAX (UNII: 2ZA36H0S2V) LEVOMENOL (UNII: 24WE03BX2T) CHOLESTEROL (UNII: 97C5T2UQ7J) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) OAT (UNII: Z6J799EAJK) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16864-040-02 1 in 1 CARTON 09/01/2021 1 56.7 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 09/01/2021 Labeler - Advantice Health, LLC (192527062)