Label: DEPO-ESTRADIOL- estradiol cypionate injection
- NDC Code(s): 0009-0271-01
- Packager: Pharmacia & Upjohn Company LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNINGS
ESTROGENS INCREASE THE RISK OF ENDOMETRIAL CANCER
Close clinical surveillance of all women taking estrogens is important. Adequate diagnostic measures including endometrial sampling, when indicated, should be undertaken to rule out malignancy in all cases of undiagnosed persistent or recurring abnormal vaginal bleeding. There is currently no evidence that the use of "natural" estrogens results in a different endometrial risk profile than "synthetic" estrogens at equivalent estrogen doses. (See WARNINGS, malignant neoplasms, Endometrial cancer.)
CARDIOVASCULAR AND OTHER RISKS
Estrogens with and without progestins should not be used for the prevention of cardiovascular disease. (See WARNINGS, Cardiovascular disorders.)
The Women's Health Initiative (WHI) study reported increased risks of myocardial infarction, stroke, invasive breast cancer, pulmonary emboli, and deep vein thrombosis in postmenopausal women (50 to 79 years of age) during 5 years of treatment with oral conjugated estrogens (CE 0.625 mg) combined with medroxyprogesterone acetate (MPA 2.5 mg) relative to placebo. (See CLINICAL PHARMACOLOGY, Clinical Studies.)
The Women's Health Initiative Memory Study (WHIMS), a substudy of WHI, reported increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 4 years of treatment with oral conjugated estrogens plus medroxyprogesterone acetate relative to placebo. It is unknown whether this finding applies to younger postmenopausal women or to women taking estrogen-alone therapy. (See CLINICAL PHARMACOLOGY, Clinical Studies.)
Other doses of conjugated estrogens with medroxyprogesterone acetate, and other combinations and dosage forms of estrogens and progestins were not studied in the WHI clinical trials and, in the absence of comparable data, these risks should be assumed to be similar. Because of these risks, estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
-
DESCRIPTION
DEPO-Estradiol Injection contains estradiol cypionate for intramuscular use. Each mL contains:
5 mg/mL—5 mg estradiol cypionate, 5.4 mg chlorobutanol anhydrous (chloral derivative) added as preservative; in 913 mg cottonseed oil.
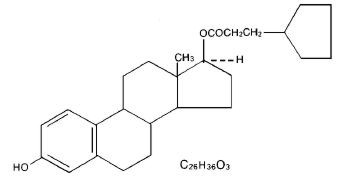
Warning: Chlorobutanol may be habit forming. The structural formula is represented below:
DEPO-Estradiol contains an oil soluble ester of estradiol 17β. The chemical name for estradiol cypionate is estradiol 17-cyclopentanepropionate.
-
CLINICAL PHARMACOLOGY
Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites, estrone and estriol, at the receptor level.
The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 mcg of estradiol daily, depending on the phase of the menstrual cycle. After menopause, most endogenous estrogen is produced by conversion of androstenedione, secreted by the adrenal cortex, to estrone by peripheral tissues. Thus, estrone and the sulfate conjugated form, estrone sulfate, are the most abundant circulating estrogens in postmenopausal women.
Estrogens act through binding to nuclear receptors in estrogen-responsive tissues. To date, two estrogen receptors have been identified. These vary in proportion from tissue to tissue.
Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH), through a negative feedback mechanism. Estrogens act to reduce the elevated levels of these hormones seen in postmenopausal women.
Absorption
When conjugated with aryl and alkyl groups for parenteral administration, the rate of absorption of oily preparations is slowed with a prolonged duration of action, such that a single intramuscular injection of estradiol valerate or estradiol cypionate is absorbed over several weeks.
Distribution
The distribution of exogenous estrogens is similar to that of endogenous estrogens. Estrogens are widely distributed in the body and are generally found in higher concentrations in the sex hormone target organs. Estrogens circulate in the blood largely bound to sex hormone binding globulin (SHBG) and albumin.
Metabolism
Exogenous estrogens are metabolized in the same manner as endogenous estrogens. Circulating estrogens exist in a dynamic equilibrium of metabolic interconversions. These transformations take place mainly in the liver. Estradiol is converted reversibly to estrone, and both can be converted to estriol, which is the major urinary metabolite. Estrogens also undergo enterohepatic recirculation via sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates into the intestine, and hydrolysis in the gut followed by reabsorption. In postmenopausal women, a significant proportion of the circulating estrogens exist as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogens.
Excretion
Estradiol, estrone, and estriol are excreted in the urine along with glucuronide and sulfate conjugates.
Drug Interactions
In vitro and in vivo studies have shown that estrogens are metabolized partially by cytochrome P450 3A4 (CYP3A4). Therefore, inducers or inhibitors of CYP3A4 may affect estrogen drug metabolism. Inducers of CYP3A4 such as St. John's Wort preparations (Hypericum perforatum), phenobarbital, carbamazepine, and rifampin may reduce plasma concentrations of estrogens, possibly resulting in a decrease in therapeutic effects and/or changes in the uterine bleeding profile. Inhibitors of CYP3A4 such as erythromycin, clarithromycin, ketoconazole, itraconazole, ritonavir and grapefruit juice may increase plasma concentrations of estrogens and may result in side effects.
Estrogen drug products administered by non oral routes are not subject to first-pass metabolism, but also undergo significant hepatic uptake, metabolism, and enterohepatic recycling.
Clinical Studies
Women's Health Initiative Studies
The Women's Health Initiative (WHI) enrolled a total of 27,000 predominantly healthy postmenopausal women to assess the risks and benefits of either the use of 0.625 mg conjugated estrogens (CE) per day alone or the use of oral 0.625 mg conjugated estrogens plus 2.5 mg medroxyprogesterone acetate (MPA) per day compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of coronary heart disease (CHD) (nonfatal myocardial infarction and CHD death), with invasive breast cancer as the primary adverse outcome studied. A "global index" included the earliest occurrence of CHD, invasive breast cancer, stroke, pulmonary embolism (PE), endometrial cancer, colorectal cancer, hip fracture, or death due to other cause. The study did not evaluate the effects of CE or CE/MPA on menopausal symptoms.
The CE/MPA substudy was stopped early because, according to the predefined stopping rule, the increased risk of breast cancer and cardiovascular events exceeded the specified benefits included in the "global index." Results of the CE/MPA substudy, which included 16,608 women (average age of 63 years, range 50 to 79; 83.9% White, 6.5% Black, 5.5% Hispanic), after an average follow-up of 5.2 years are presented in Table 1 below:
Table 1: RELATIVE AND ABSOLUTE RISK SEEN IN THE CE/MPA SUBSTUDY OF WHI * Event † Relative Risk
CE/MPA vs placebo
at 5.2 Years
(95% CI‡)Placebo
n = 8102CE/MPA
n = 8506Absolute Risk per 10,000 Person-years - *
- adapted from JAMA, 2002; 288:321–333
- †
- a subset of the events was combined in a "global index," defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, endometrial cancer, colorectal cancer, hip fracture, or death due to other causes
- ‡
- nominal confidence intervals unadjusted for multiple looks and multiple comparisons
- §
- includes metastatic and non-metastatic breast cancer with the exception of in situ breast cancer
- ¶
- not included in Global Index
CHD events
1.29 (1.02–1.63)
30
37
Non-fatal MI
1.32 (1.02–1.72)
23
30
CHD death
1.18 (0.70–1.97)
6
7
Invasive breast cancer §
1.26 (1.00–1.59)
30
38
Stroke
1.41 (1.07–1.85)
21
29
Pulmonary embolism
2.13 (1.39–3.25)
8
16
Colorectal cancer
0.63 (0.43–0.92)
16
10
Endometrial cancer
0.83 (0.47–1.47)
6
5
Hip fracture
0.66 (0.45–0.98)
15
10
Death due to causes other than the events above
0.92 (0.74–1.14)
40
37
Global index †
1.15 (1.03–1.28)
151
170
Deep vein thrombosis ¶
2.07 (1.49–2.87)
13
26
Vertebral fractures ¶
0.66 (0.44–0.98)
15
9
Other osteoporotic fractures ¶
0.77 (0.69–0.86)
170
131
For those outcomes included in the "global index," the absolute excess risks per 10,000 person-years in the group treated with CE/MPA were 7 more CHD events, 8 more strokes, 8 more PEs, and 8 more invasive breast cancers, while absolute risk reductions per 10,000 person-years were 6 fewer colorectal cancers and 5 fewer hip fractures. The absolute excess risk of events included in the "global index" was 19 per 10,000 women-years. There was no difference between the groups in terms of all-cause mortality. (See BOXED WARNINGS, WARNINGS and PRECAUTIONS.)
Women's Health Initiative Memory Study
The Women's Health Initiative Memory Study (WHIMS), a substudy of WHI, enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47% were age 65 to 69 years, 35% were 70 to 74 years, and 18% were 75 years of age and older) to evaluate the effects of CE/MPA (0.625 mg conjugated estrogens plus 2.5 mg medroxyprogesterone acetate) on the incidence of probable dementia (primary outcome) compared with placebo.
After an average follow-up of 4 years, 40 women in the estrogen/progestin group (45 per 10,000 women-years) and 21 in the placebo group (22 per 10,000 women-years) were diagnosed with probable dementia. The relative risk of probable dementia in the hormone therapy group was 2.05 (95% CI, 1.21 to 3.48) compared to placebo. Differences between groups became apparent in the first year of treatment. It is unknown whether these findings apply to younger postmenopausal women. (See BOXED WARNINGS and WARNINGS, Dementia.)
Comparative clinical studies have demonstrated that estradiol cypionate produces estrogenic effects that are qualitatively the same as those produced by other estradiol esters. In menopausal women, the average duration of estrogenic effect (as measured by vaginal smear) following a single injection of 5 mg of estradiol cypionate was found to be approximately 3 to 4 weeks. Relief of vasomotor symptoms was observed to occur within 1 to 5 days and to be maintained for 1 to 8 weeks, with an average of approximately 5 weeks.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Estrogens should not be used in individuals with any of the following conditions:
- 1.
- Undiagnosed abnormal genital bleeding.
- 2.
- Known or suspected cancer of the breast.
- 3.
- Known or suspected estrogen-dependent neoplasia.
- 4.
- Active deep vein thrombosis, pulmonary embolism or history of these conditions.
- 5.
- Active or recent (e.g., within the past year) arterial thromboembolic disease (e.g., stroke, myocardial infarction).
- 6.
- Liver dysfunction or disease.
- 7.
- DEPO-Estradiol should not be used in patients with known hypersensitivity to its ingredients.
- 8.
- Known or suspected pregnancy. There is no indication for DEPO-Estradiol in pregnancy.
There appears to be little or no increased risk of birth defects in children born to women who have used estrogens and progestins from oral contraceptives inadvertently during early pregnancy. (See PRECAUTIONS.)
-
WARNINGS
See BOXED WARNINGS
1. Cardiovascular disorders
Estrogen and estrogen/progestin therapy have been associated with an increased risk of cardiovascular events such as myocardial infarction and stroke, as well as venous thrombosis and pulmonary embolism (venous thromboembolism or VTE). Should any of these occur or be suspected, estrogens should be discontinued immediately.
Risk factors for arterial vascular disease (e.g., hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (e.g., personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
a. Coronary heart disease and stroke
In the Women's Health Initiative (WHI) study, an increase in the number of myocardial infarctions and strokes has been observed in women receiving CE compared to placebo. These observations are preliminary, and the study is continuing. (See CLINICAL PHARMACOLOGY, Clinical Studies.)
In the CE/MPA substudy of WHI, an increased risk of coronary heart disease (CHD) events (defined as nonfatal myocardial infarction and CHD death) was observed in women receiving CE/MPA compared to women receiving placebo (37 vs. 30 per 10,000 women-years). The increase in risk was observed in year one and persisted.
In the same substudy of WHI, an increased risk of stroke was observed in women receiving CE/MPA compared to women receiving placebo (29 vs. 21 per 10,000 women-years). The increase in risk was observed after the first year and persisted.
In postmenopausal women with documented heart disease (n = 2,763, average age 66.7 years) a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS) treatment with CE/MPA (0.625 mg/2.5 mg per day) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE/MPA did not reduce the overall rate of CHD events in postmenopausal women with established coronary heart disease. There were more CHD events in the CE/MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred and twenty one women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE/MPA group and the placebo group in HERS, HERS II, and overall.
Large doses of estrogen (5 mg conjugated estrogens per day), comparable to those used to treat cancer of the prostate and breast, have been shown in a large prospective clinical trial in men to increase the risks of nonfatal myocardial infarction, pulmonary embolism, and thrombophlebitis.
b. Venous thromboembolism (VTE)
In the Women's Health Initiative (WHI) study, in women receiving CE compared to placebo, the risk of VTE (including both DVT and PE) was increased 33% (28 vs. 21 per 10,000 person-years) although only the increased rate of DVT reached statistical significance (p = 0.03). (See CLINICAL PHARMACOLOGY, Clinical Studies.)
In the CE/MPA treatment substudy of WHI, a 2-fold greater rate of VTE, including deep venous thrombosis and pulmonary embolism, was observed in women receiving treatment with CE/MPA compared to women receiving placebo. The rate of VTE was 34 per 10,000 woman-years in the CE/MPA group compared to 16 per 10,000 woman-years in the placebo group. The increase in VTE risk was observed during the first year and persisted.
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
2. Malignant neoplasms
a. Endometrial cancer
The use of unopposed estrogens in women with intact uteri has been associated with an increased risk of endometrial cancer. The reported endometrial cancer risk among unopposed estrogen users was about 2-to-12-fold greater than in non-users, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with use of estrogens for less than one year. The greatest risk appears associated with prolonged use, with increased risks of 15-to-24-fold for five to ten years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women taking estrogen/progestin combinations is important. Adequate diagnostic measures, including endometrial sampling when indicated, should be undertaken to rule out malignancy in all cases of undiagnosed persistent or recurring abnormal vaginal bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
b. Breast cancer
The most important randomized clinical trial providing information about breast cancer in estrogen plus progestin users is the WHI substudy of daily CE (0.625 mg) plus MPA (2.5 mg). After a mean follow-up of 5.6 years the estrogen plus progestin substudy reported an increased risk of invasive breast cancer in women who took daily CE plus MPA. In this substudy, prior use of estrogen-alone or estrogen plus progestin therapy was reported by 26 percent of the women. The relative risk of invasive breast cancer was 1.24, and the absolute risk was 41 versus 33 cases per 10,000 women-years, for CE plus MPA compared with placebo. Among women who reported prior use of hormone therapy, the relative risk of invasive breast cancer was 1.86, and the absolute risk was 46 versus 25 cases per 10,000 women-years, for CE plus MPA compared with placebo. Among women who reported no prior use of hormone therapy, the relative risk of invasive breast cancer was 1.09, and the absolute risk was 40 versus 36 cases per 10,000 women-years, for CE plus MPA compared with placebo. In the same substudy, invasive breast cancers were larger, were more likely to be node positive, and were diagnosed at a more advanced stage in the CE (0.625 mg) plus MPA (2.5 mg) group compared with the placebo group. Metastatic disease was rare with no apparent difference between the two groups. Other prognostic factors such as histologic subtype, grade and hormone receptor status did not differ between groups.
The most important randomized clinical trial providing information about breast cancer in estrogen-alone users is the WHI substudy of daily CE (0.625 mg)-alone. In the WHI estrogen-alone substudy, after an average follow-up of 7.1 years, daily CE (0.625 mg) alone was not associated with an increased risk of invasive breast cancer [relative risk (RR) 0.80].
Consistent with the Women’s Health Initiative (WHI clinical trials), observational studies have also reported an increased risk of breast cancer for estrogen plus progestin therapy and a smaller, but still increased risk, for estrogen-alone therapy after several years of use. One large meta-analysis of prospective cohort studies reported increased risks that were dependent upon duration of use and could last up to >10 years after discontinuation of estrogen plus progestin therapy and estrogen-alone therapy. Extension of the WHI trials also demonstrated increased breast cancer risk associated with estrogen plus progestin therapy. Observational studies also suggest that the risk of breast cancer was greater, and became apparent earlier, with estrogen plus progestin therapy as compared to the risk with estrogen-alone therapy. However, these studies have not found significant variation in the risk of breast cancer among different estrogen plus progestin combinations, doses, or routes of administration.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation. All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations.
In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
3. Dementia
In the Women's Health Initiative Memory Study (WHIMS), 4,532 generally healthy postmenopausal women 65 years of age and older were studied, of whom 35% were 70 to 74 years of age and 18% were 75 or older. After an average follow-up of 4 years, 40 women being treated with CE/MPA (1.8%, n= 2,229) and 21 women in the placebo group (0.9%, n= 2,303) received diagnoses of probable dementia. The relative risk for CE/MPA versus placebo was 2.05 (95% confidence interval 1.21 – 3.48), and was similar for women with and without histories of menopausal hormone use before WHIMS. The absolute risk of probable dementia for CE/MPA versus placebo was 45 versus 22 cases per 10,000 women-years. It is unknown whether these findings apply to younger postmenopausal women. (See CLINICAL PHARMACOLOGY, Clinical Studies and PRECAUTIONS, Geriatric Use.)
4. Gallbladder disease
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogen has been reported.
5. Hypercalcemia
Estrogen administration may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If hypercalcemia occurs, use of the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
6. Visual abnormalities
Retinal vascular thrombosis has been reported in patients receiving estrogens. Discontinue medication pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, estrogens should be permanently discontinued.
-
PRECAUTIONS
A. General
1. Addition of progestin when a woman has not had a hysterectomy
Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration, or daily with estrogen in a continuous regimen, have reported a lowered incidence of endometrial hyperplasia than would be induced by estrogen treatment alone. Endometrial hyperplasia may be a precursor to endometrial cancer.
There are, however, possible risks that may be associated with the use of progestins with estrogens compared to estrogen-alone regimens. These include a possible increased risk of breast cancer, adverse effects on lipoprotein metabolism (e.g., lowering HDL, raising LDL) and impairment of glucose tolerance.
2. Elevated blood pressure
In a small number of case reports, substantial increases in blood pressure have been attributed to idiosyncratic reactions to estrogens. In a large, randomized, placebo-controlled clinical trial, a generalized effect of estrogens on blood pressure was not seen. Blood pressure should be monitored at regular intervals with estrogen use.
3. Hypertriglyceridemia
In patients with pre-existing hypertriglyceridemia, estrogen therapy may be associated with elevations of plasma triglycerides leading to pancreatitis and other complications.
4. Impaired liver function and past history of cholestatic jaundice
Estrogens may be poorly metabolized in patients with impaired liver function. For patients with a history of cholestatic jaundice associated with past estrogen use or with pregnancy, caution should be exercised and in the case of recurrence, medication should be discontinued.
5. Hypothyroidism
Estrogen administration leads to increased thyroid-binding globulin (TBG) levels. Patients with normal thyroid function can compensate for the increased TBG by making more thyroid hormone, thus maintaining free T4 and T3 serum concentrations in the normal range. Patients dependent on thyroid hormone replacement therapy who are also receiving estrogens may require increased doses of their thyroid replacement therapy. These patients should have their thyroid function monitored in order to maintain their free thyroid hormone levels in an acceptable range.
6. Fluid retention
Because estrogens may cause some degree of fluid retention, patients with conditions that might be influenced by this factor, such as a cardiac or renal dysfunction, require careful observation when estrogens are prescribed.
8. Ovarian cancer
The CE/MPA substudy of WHI reported that estrogen plus progestin increased the risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE/MPA versus placebo was 1.58 (95% confidence interval 0.77 – 3.24) but was not statistically significant. The absolute risk for CE/MPA versus placebo was 20 versus 12 cases per 10,000 women-years. In some epidemiologic studies, the use of estrogen alone, in particular for ten or more years, has been associated with an increased risk of ovarian cancer. Other epidemiologic studies have not found these associations.
9. Exacerbation of endometriosis
Endometriosis may be exacerbated with administration of estrogens. A few cases of malignant transformation of residual endometrial implants have been reported in women treated post-hysterectomy with estrogen-alone therapy. For patients known to have residual endometriosis post-hysterectomy, the addition of progestin should be considered.
B. PATIENT INFORMATION
Physicians are advised to discuss the PATIENT INFORMATION leaflet with patients for whom they prescribe DEPO-Estradiol.
C. LABORATORY TESTS
Estrogen administration should be initiated at the lowest dose for the approved indication and then guided by clinical response, rather than by serum hormone levels (e.g., estradiol, FSH).
D. DRUG/LABORATORY TEST INTERACTIONS
- 1.
- Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, II-VII-X complex, and beta-thromboglobulin; decreased levels of anti-factor Xa and antithrombin III, decreased antithrombin III activity; increased levels of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
- 2.
- Increased thyroid-binding globulin (TBG) levels leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI), T4 levels (by column or by radioimmunoassay) or T3 levels by radioimmunoassay. T3 resin uptake is decreased, reflecting the elevated TBG. Free T4 and free T3 concentrations are unaltered. Patients on thyroid replacement therapy may require higher doses of thyroid hormone.
- 3.
- Other binding proteins may be elevated in serum, i.e., corticosteroid binding globulin (CBG), sex-hormone binding globulin (SHBG), leading to increased circulating corticosteroids and sex steroids, respectively. Free or biologically active hormone levels concentrations are unchanged. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-1-anti-trypsin, ceruloplasmin).
- 4.
- Increased plasma HDL and HDL-2 subfraction concentrations, reduced LDL cholesterol concentration, increased triglycerides levels.
- 5.
- Impaired glucose tolerance.
- 6.
- Reduced response to metyrapone test.
- 7.
- Reduced serum folate concentration.
E. CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
Long-term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas of the breast, uterus, cervix, vagina, testis, and liver. (See BOXED WARINGS, WARNINGS and PRECAUTIONS.)
F. PREGNANCY
DEPO-Estradiol should not be used during pregnancy. See CONTRAINDICATIONS and Boxed WARNINGS.
G. NURSING MOTHERS
Estrogen administration to nursing mothers has been shown to decrease the quantity and quality of the milk. Detectable amounts of estrogens have been identified in the milk of mothers receiving this drug. Caution should be exercised when DEPO-Estradiol is administered to a nursing woman.
H. GERIATRIC USE
In the Women's Health Initiative Memory Study, including 4,532 women 65 years of age and older, followed for an average of 4 years, 82% (n= 3,729) were 65 to 74 while 18% (n= 803) were 75 and over. Most women (80%) had no prior hormone therapy use. Women treated with conjugated estrogens plus medroxyprogesterone acetate were reported to have a two-fold increase in the risk of developing probable dementia. Alzheimer's disease was the most common classification of probable dementia in both the conjugated estrogens plus medroxyprogesterone acetate group and the placebo group. Ninety percent of the cases of probable dementia occurred in the 54% of women that were older than 70. (See WARNINGS, Dementia.)
-
ADVERSE REACTIONS
See BOXED WARNINGS, WARNINGS and PRECAUTIONS.
The following additional adverse reactions have been reported with estrogens and/or progestin therapy.
- 1.
-
Genitourinary system
Changes in vaginal bleeding pattern and abnormal withdrawal bleeding or flow; breakthrough bleeding, spotting; dysmenorrhea; increase in size of uterine leiomyomata; vaginitis including vaginal candidiasis; change in amount of cervical secretion; changes in cervical ectropion; ovarian cancer; endometrial hyperplasia; endometrial cancer. - 2.
-
Breasts
Tenderness, enlargement pain, nipple discharge, galactorrhea; fibrocystic breast changes; breast cancer. - 3.
-
Cardiovascular
Deep and superficial venous thrombosis; pulmonary embolism; thrombophlebitis; myocardial infarction; stroke; increase in blood pressure. - 4.
-
Gastrointestinal
Nausea, vomiting; abdominal cramps, bloating; cholestatic jaundice; increased incidence of gallbladder disease; pancreatitis, enlargement of hepatic hemangiomas. - 5.
-
Skin
Chloasma or melasma that may persist when drug is discontinued. Erythema multiforme; erythema nodosum; hemorrhagic eruption; loss of scalp hair; hirsutism; pruritus, rash. - 6.
-
Eyes
Retinal vascular thrombosis; steepening of corneal curvature; intolerance to contact lenses. - 7.
-
Central nervous system
Headache, migraine, dizziness; mental depression; chorea; nervousness; mood disturbances; irritability; exacerbation of epilepsy, dementia. - 8.
-
Miscellaneous
Increase or decrease in weight; reduced carbohydrate tolerance; aggravation of porphyria; edema; changes in libido; arthralgias; leg cramps; anaphylactoid/anaphylactic reactions including urticaria and angioedema; hypocalcemia; exacerbation of asthma; increased triglycerides.
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Warming and shaking the vial should redissolve any crystals that may have formed during storage at temperatures lower than recommended.
DEPO-Estradiol INJECTION IS FOR INTRAMUSCULAR USE ONLY.
When estrogen is prescribed for a woman with a uterus, progestin should also be initiated to reduce the risk of endometrial cancer. A woman without a uterus does not need progestin. Use of estrogen, alone or in combination with a progestin, should be with the lowest effective dose and for the shortest duration consistent with treatment goals and risks for the individual woman. Patients should be reevaluated periodically as clinically appropriate (e.g., 3-month to 6-month intervals) to determine if treatment is still necessary. (See BOXED WARNINGS and WARNINGS.) For women who have a uterus, adequate diagnostic measures, such as endometrial sampling, when indicated, should be undertaken to rule out malignancy in cases of undiagnosed persistent or recurring abnormal vaginal bleeding.
- 1.
- Short-term cyclic use for treatment of moderate to severe vasomotor symptoms, vulval and vaginal atrophy associated with the menopause, the lowest dose and regimen that will control symptoms should be chosen and medication should be discontinued as promptly as possible.
Attempts to discontinue or taper medication should be made at 3- to 6-month intervals. The usual dosage range is 1 to 5 mg injected every 3 to 4 weeks. - 2.
- For treatment of female hypoestrogenism due to hypogonadism 1.5 to 2 mg injected at monthly intervals.
- HOW SUPPLIED
-
REFERENCES
- 1.
- Ziel HK, Finkle WD: Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med 293:1167–1170, 1975.
- 2.
- Smith DC, Prentice R, Thompson DJ, et al: Association of exogenous estrogen and endometrial carcinoma. N Engl J Med 293:1164–1167, 1975.
- 3.
- Mack TM, Pike MC, Henderson BE, et al: Estrogens and endometrial cancer in a retirement community. N Engl J Med 294:1262–1267, 1976.
- 4.
- Weiss NS, Szekely DR, Austin DF: Increasing incidence of endometrial cancer in the United States. N Engl J Med 294:1259–1262, 1976.
- 5.
- Herbst AL, Ulfelder H, Poskanzer DC: Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med 284:878–881, 1971.
- 6.
- Greenwald P, Barlow JJ, Nasca PC, Burnett WS: Vaginal cancer after maternal treatment with synthetic estrogens. N Engl J Med 285:390–392, 1971.
- 7.
- Lanier AP, Noller KL, Decker DG, Elveback LR, Kurland LT: Cancer and stilbestrol. A follow-up of 1,719 persons exposed to estrogens in utero and born 1943–1959. Mayo Clin Proc 48:793–799, 1973.
- 8.
- Herbst AL, Kurman RJ, Scully RE: Vaginal and cervical abnormalities after exposure to stilbestrol in utero. Obstet Gynecol 40:287–298, 1972.
- 9.
- Herbst AL, Robboy SJ, Macdonald GJ, Scully RE: The effects of local progesterone on stilbestrol-associated vaginal adenosis. Am J Obstet Gynecol 118:607–615, 1974.
- 10.
- Herbst AL, Poskanzer DC, Robboy SJ, Friedlander L, Scully RE: Prenatal exposure to stilbestrol. A prospective comparison of exposed female offspring with unexposed control. N Engl J Med 292:334–339, 1975.
- 11.
- Stafl A, Mattingly RF, Foley DV, Fetherston WC: Clinical diagnosis of vaginal adenosis. Obstet Gynecol 43:118–128, 1974.
- 12.
- Sherman AL, Goldrath M, Berlin A, et al: Cervical-vaginal adenosis after in utero exposure to synthetic estrogens. Obstet Gynecol 44:531545, 1974.
- 13.
- Gall, Kirman B, Stern J: Hormonal pregnancy tests and congenital malformation. Nature 216:83, 1967.
- 14.
- Levy EP, Cohen A, Fraser FC: Hormone treatment during pregnancy and congenital heart defects. Lancet 1:611, 1973.
- 15.
- Nora JJ, Nora AH: Birth defects and oral contraceptives. Lancet 1:941–942, 1973.
- 16.
- Janerich DT, Piper JM, Glebatis DM: Oral contraceptives and congenital limb-reduction defects. N Engl J Med 291:697–700, 1974.
- 17.
- Boston Collaborative Drug Surveillance Program: Surgically confirmed gall bladder disease, venous thromboembolism, and breast tumors in relation to post-menopausal estrogen therapy. N Engl J Med 290:15–19, 1974.
- 18.
- Hoover R, Gray LA, Cole P, MacMahon B: Menopausal estrogens and breast cancer. N Engl J Med 295:401–405, 1976.
- 19.
- Boston Collaborative Drug Surveillance Program: Oral contraceptives and venous thromboembolic disease, surgically confirmed gall bladder disease, and breast tumors. Lancet 1:1399–1404, 1973.
- 20.
- Daniel DG, Campbell H, Turnbull AC: Puerperal thromboembolism and suppression of lactation. Lancet 2:287–289, 1967.
- 21.
- The Veterans Administration Cooperative Urological Research Group: Carcinoma of the prostate: Treatment comparisons. J Urol 98:516522, 1967.
- 22.
- Bailar JC: Thromboembolism and estrogen therapy. Lancet 2:560, 1967.
- 23.
- Blackard CE, Doe RP, Mellinger GT, Byar DP: Incidence of cardiovascular disease and death in patients receiving diethylstilbestrol for carcinoma of the prostate. Cancer 26:249–256, 1970.
- 24.
- Royal College of General Practitioners: Oral contraception and thromboembolic disease. J R Coll Gen Pract 13:267–279, 1967.
- 25.
- Inman WHW, Vessey MP: Investigation of deaths from pulmonary, coronary, and cerebral thrombosis and embolism in women of childbearing age. Br Med J 2:193–199, 1968.
- 26.
- Vessey MP, Doll R: Investigation of relation between use of oral contraceptives and thromboembolic disease. A further report. Br Med J 2:651–657, 1969.
- 27.
- Sartwell PE, Masi AT, Arthes FG, et al: Thromboembolism and oral contraceptives: An epidemiologic case-control study. Am J Epidemiol 90:365–380, 1969.
- 28.
- Collaborative Group for the Study of Stroke in Young Women: Oral contraception and increased risk of cerebral ischemia or thrombosis. N Engl J Med 288:871–878, 1973.
- 29.
- Collaborative Group for the Study of Stroke in Young Women: Oral contraceptives and stroke in young women: Associated risk factors. JAMA 231:718–722, 1975.
- 30.
- Mann JI, Inman WHW: Oral contraceptives and death from myocardial infarction. Br Med J 2:245–248, 1975.
- 31.
- Mann JI, Vessey MP, Thorogood M, Doll R: Myocardial infarction in young women with special reference to oral contraceptive practice. Br Med J 2:241–245, 1975.
- 32.
- Inman WHW, Vessey MP, Westerholm B, Engelund A: Thromboembolic disease and the steroidal content of oral contraceptives. Br Med J 2:203–209, 1970.
- 33.
- Stolley PD, Tonascia JA, Tockman MS, et al: Thrombosis with low-estrogen oral contraceptives. Am J Epidemiol 102:197–208, 1975.
- 34.
- Vessey MP, Doll R, Fairbairn AS, Glober G: Postoperative thromboembolism and the use of oral contraceptives. Br Med J 3:123–126, 1970.
- 35.
- Greene GR, Sartwell PE: Oral contraceptive use in patients with thromboembolism following surgery, trauma or infection. Am J Public Health 62:680–685, 1972.
- 36.
- Rosenberg L, Armstrong B, Phil D, Jick H: Myocardial infarction and estrogen therapy in post-menopausal women. N Engl J Med 294:1256–1259, 1976.
- 37.
- Coronary Drug Project Research Group: The Coronary Drug Project: Initial findings leading to modifications of its research protocol. JAMA 214:1303–1313, 1970.
- 38.
- Baum J, Holtz F, Bookstein JJ, Klein EW: Possible association between benign hepatomas and oral contraceptives. Lancet 2:926–929, 1973.
- 39.
- Mays ET, Christopherson WM, Mahr MM, Williams HC: Hepatic changes in young women ingesting contraceptive steroids. Hepatic hemorrhage and primary hepatic tumors. JAMA 235:730–732, 1976.
- 40.
- Edmondson HA, Henderson B, Benton B: Liver-cell adenomas associated with use of oral contraceptives. N Engl J Med 294:470–472, 1976.
- 41.
- Pfeffer RI, VanDenNoort S: Estrogen use and stroke risk in post-menopausal women. Am J Epidemiol 103:445–456, 1976.
- SPL UNCLASSIFIED SECTION
-
PATIENT INFORMATION
DEPO-Estradiol®
Brand of estradiol cypionate injection, USP
Read this PATIENT INFORMATION before you start taking DEPO-Estradiol and read what you get each time you refill DEPO-Estradiol. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
WHAT IS THE MOST IMPORTANT INFORMATION I SHOULD KNOW ABOUT DEPO-ESTRADIOL (AN ESTROGEN HORMONE)?
Estrogens increase the chances of getting cancer of the uterus.
Report any unusual vaginal bleeding right away while you are taking estrogens. Vaginal bleeding after menopause may be a warning sign of cancer of the uterine (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause.
Do not use estrogens with or without progestins to prevent heart disease, heart attacks, or strokes.
Using estrogens with or without progestins may increase your chances of getting heart attacks, strokes, breast cancer, and blood clots. You and your healthcare provider should talk regularly about whether you still need treatment with DEPO-Estradiol.
What is DEPO-Estradiol?Depo-Estradiol injection is an estrogen product. The information below is that which the U.S. Food and Drug Administration requires be provided for all patients taking estrogens. For further information ask your doctor.
What is DEPO-Estradiol used for?DEPO-Estradiol is used during and after menopause to:
- •
-
reduce moderate or severe menopausal symptoms. Estrogens are hormones made by a woman's ovaries. The ovaries normally stop making estrogens when a woman is between 45 to 55 years old. This drop in body estrogen levels causes the "change of life" or menopause (end of monthly menstrual periods). Sometimes both ovaries are removed during an operation before natural menopause takes place, the sudden drop in estrogen levels causes "surgical menopause."
When the estrogen levels begin dropping, some women develop very uncomfortable symptoms, such as feeling of warmth in the face, neck and chest or sudden strong feelings of heat and sweating ("hot flashes" or "hot flushes"). Using estrogen drugs can help the body adjust to lower estrogen levels and reduce these symptoms. Most women have only mild menopause symptoms or none at all and do not need estrogen drugs for these symptoms. - •
-
treat moderate to severe itching, burning, and dryness in or around the vagina.
You and your healthcare provider should talk regularly about whether you still need treatment with DEPO-Estradiol to control these problems.
DEPO-Estradiol is also used to:- •
- treat certain conditions in women before menopause if their ovaries do not make enough estrogen.
Who should not take DEPO-Estradiol?Do not start taking DEPO-Estradiol if you:
- •
- have unusual vaginal bleeding.
- •
-
currently have or have had certain cancers.
Estrogens may increase the chances of getting certain types of cancers, including cancer of the breast or uterus. If you have or had cancer, talk with your healthcare provider about whether you should take DEPO-Estradiol. - •
- had a stroke or heart attack in the past year.
- •
- currently have or have had blood clots.
- •
- are allergic to DEPO-Estradiol or any of its ingredients.
See the end of this leaflet for a list of ingredients in DEPO-Estradiol.
- •
- think you may be pregnant.
Tell your healthcare provider:
- •
- if you are breastfeeding.
The hormone in DEPO-Estradiol can pass into your milk.
- •
-
about all of your medical problems.
Your healthcare provider may need to check you more carefully if you have certain medical conditions, such as asthma (wheezing), epilepsy (seizures), diabetes, migraine, endometriosis, lupus, angioedema (swelling of face and tongue), hypertension (high blood pressure), problems with your heart, liver, thyroid, kidneys, or have high calcium levels in your blood. - •
-
about all the medicines you take.
This includes prescription and nonprescription medicines, vitamins, and herbal supplements. Some medicines may affect how DEPO-ESTRADIOL works. DEPO-ESTRADIOL may also affect how your other medicines work. - •
-
if you are going to have surgery or will be on bed rest.
You may need to stop taking estrogens.
How should I take DEPO-Estradiol?Take DEPO-Estradiol as directed by your healthcare provider.
Estrogens should be used only as long as needed. You and your healthcare provider should talk regularly (for example, every 3 to 6 months) about whether you still need treatment with DEPO-ESTRADIOL.
What are the possible side effects of estrogens?Less common but serious side effects include:
- •
- Breast cancer
- •
- Cancer of the uterus
- •
- Stroke
- •
- Heart attack
- •
- Blood clots
- •
- Gallbladder disease
- •
- Ovarian cancer
These are some of the warning signs of serious side effects:
- •
- Breast lumps
- •
- Unusual vaginal bleeding
- •
- Dizziness and faintness
- •
- Changes in speech
- •
- Severe headaches
- •
- Chest pain
- •
- Shortness of breath
- •
- Pains in your legs
- •
- Changes in vision
- •
- Vomiting
Call your healthcare provider right away if you get any of these warning signs, or any other unusual symptom that concerns you.
Common side effects include:
- •
- Headache
- •
- Breast pain
- •
- Irregular vaginal bleeding or spotting
- •
- Stomach/abdominal cramps, bloating
- •
- Nausea and vomiting
Other side effects include:
- •
- High blood pressure
- •
- Liver problems
- •
- High blood sugar
- •
- Fluid retention
- •
- Enlargement of benign tumors of the uterus ("fibroids")
- •
- Vaginal yeast infections
- •
- Hair loss
These are not all the possible side effects of DEPO-Estradiol. For more information, ask your healthcare provider or pharmacist.
What can I do to lower my chances of getting a serious side effect with DEPO-Estradiol?- •
- Talk with your healthcare provider regularly about whether you should continue taking DEPO-ESTRADIOL. If you have a uterus, talk to your healthcare provider about whether the addition of a progestin is right for you. See your healthcare provider right away if you get vaginal bleeding while taking DEPO-ESTRADIOL. Have a breast exam and mammogram (breast X-ray) every year unless your healthcare provider tells you something else. If members of your family have had breast cancer or if you have ever had breast lumps or an abnormal mammogram, you may need to have breast examinations more often. If you have high blood pressure, high cholesterol (fat in the blood), diabetes, are overweight, or if you use tobacco, you may have higher chances for getting heart disease. Ask your healthcare provider for ways to lower your chances for getting heart disease.
General information about safe and effective use of DEPO-EstradiolMedicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not take DEPO-Estradiol for conditions for which it was not prescribed. Do not give DEPO-Estradiol to other people, even if they have the same symptoms you have. It may harm them. Keep DEPO-Estradiol out of the reach of children.
This leaflet provides a summary of the most important information about DEPO-Estradiol. If you would like more information, talk with your healthcare provider or pharmacist. You can ask for information about DEPO-Estradiol that is written for health professionals. You can get more information by calling the toll free number 1-800-438-1985. You are cautioned to discuss very carefully with your doctor or healthcare provider all the possible risks and benefits of long-term estrogen and progestin treatment as they affect you personally.
- PRINCIPAL DISPLAY PANEL - 5 mg/mL Vial Label
- PRINCIPAL DISPLAY PANEL - 5 mg/mL Vial Carton
-
INGREDIENTS AND APPEARANCE
DEPO-ESTRADIOL
estradiol cypionate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0009-0271 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESTRADIOL CYPIONATE (UNII: 7E1DV054LO) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL CYPIONATE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength COTTONSEED OIL (UNII: H3E878020N) 913 mg in 1 mL CHLOROBUTANOL (UNII: HM4YQM8WRC) 5.4 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0009-0271-01 1 in 1 CARTON 08/15/1979 1 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA085470 08/15/1979 Labeler - Pharmacia & Upjohn Company LLC (618054084) Establishment Name Address ID/FEI Business Operations Pharmacia & Upjohn Company LLC 618054084 ANALYSIS(0009-0271) , API MANUFACTURE(0009-0271) , LABEL(0009-0271) , MANUFACTURE(0009-0271) , PACK(0009-0271)