Label: ALLERGY RELIEF- fexofenadine hydrochloride tablet, film coated
- NDC Code(s): 0363-1847-39, 0363-1847-49, 0363-1847-75, 0363-1847-95
- Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- •
- do not take more than directed
- •
- do not take at the same time as aluminum or magnesium antacids
- •
- do not take with fruit juices (see Directions)
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
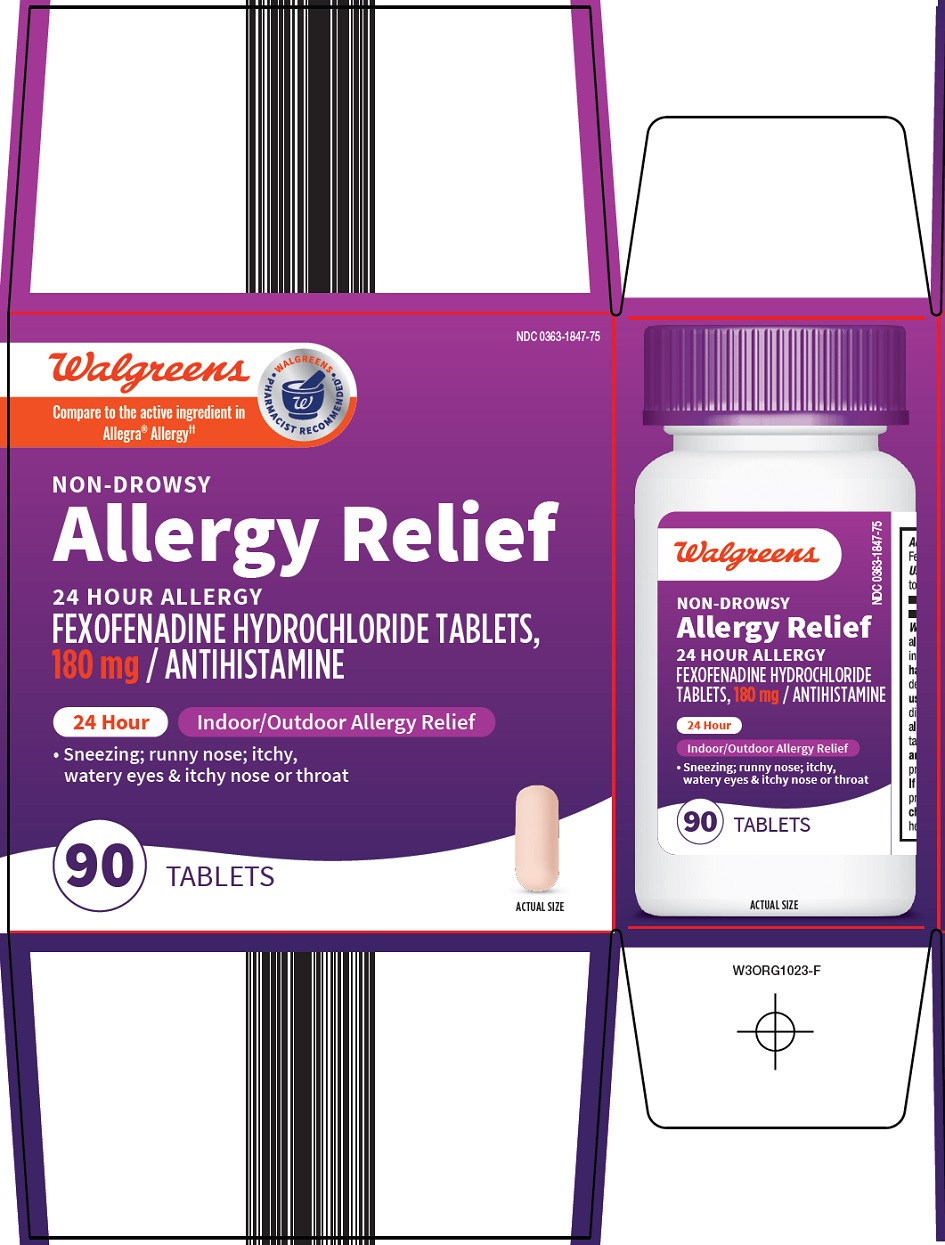
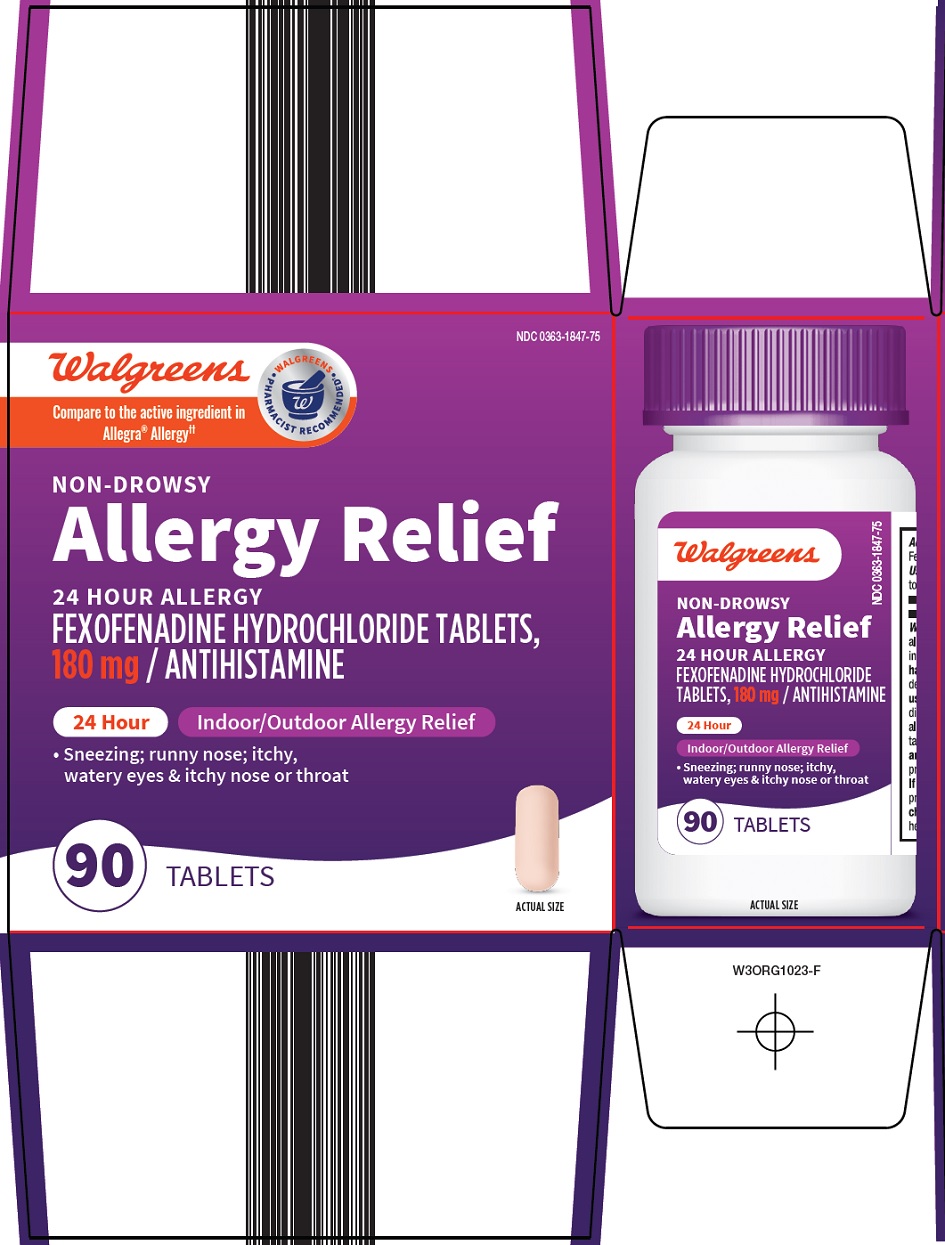
Package/Label Principal Display Panel
Walgreens
Compare to the active ingredient in Allegra® Allergy
WALGREENS PHARMACIST RECOMMENDED
NON-DROWSY
Allergy Relief
24 HOUR ALLERGY
FEXOFENADINE HYDROCHLORIDE TABLETS, 180 mg / ANTIHISTAMINE
24 Hour
Indoor/Outdoor Allergy Relief
• Sneezing; runny nose; itchy, watery eyes & itchy nose or throat
90 TABLETS
ACTUAL SIZE
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
fexofenadine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-1847 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PINK Score no score Shape OVAL Size 18mm Flavor Imprint Code L847 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-1847-39 1 in 1 CARTON 02/01/2024 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:0363-1847-75 1 in 1 CARTON 02/01/2024 2 90 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:0363-1847-95 1 in 1 CARTON 02/01/2024 3 45 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:0363-1847-49 1 in 1 CARTON 12/13/2024 4 40 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212971 02/01/2024 Labeler - Walgreen Company (008965063)