Label: SODIUM CHLORIDE irrigant
- NDC Code(s): 0338-0110-01, 0338-0110-04
- Packager: Baxter Healthcare Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Health Care Provider Letter
Please refer to the FDA-approved prescribing information for the drug product listed below:
• 0.9% Sodium Chloride Irrigation USP (click https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=886f889b-f3cf-4197-b307-6cfa88c89436)• Complete and submit the report Online: www.fda.gov/medwatch/report.htm
• Regular mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178 (1-800-332-0178).To report productqualityissues associated with this imported product, please contact Baxter Product Surveillance through Baxter Product Feedback Portal (https://productfeedback.baxter.com/)
-
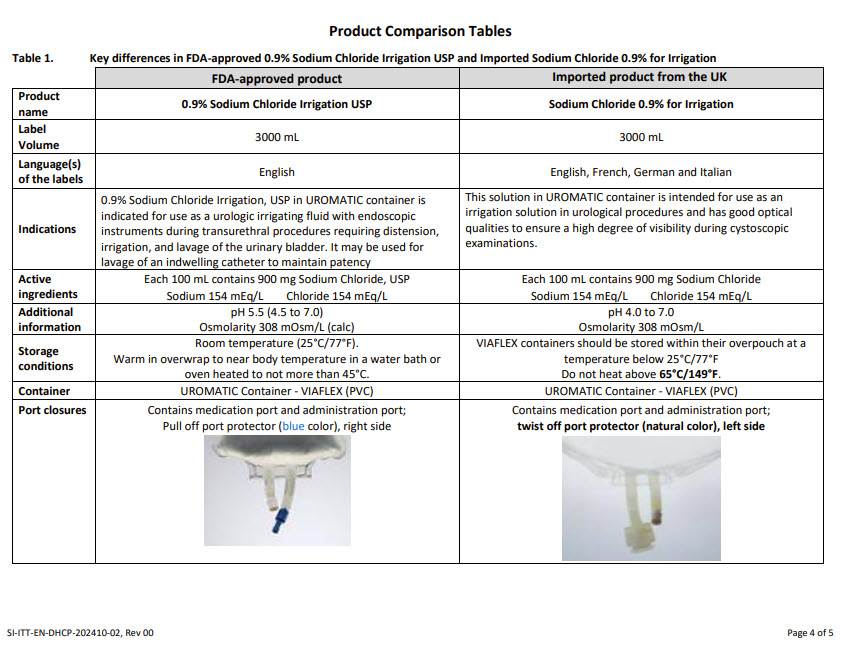
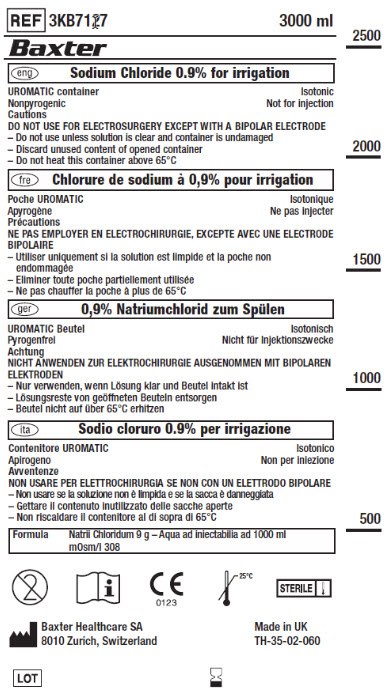
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Container Label
REF 3KB7127 3000 ml
Baxter Logo
eng Sodium Chloride 0.9% for irrigation
UROMATIC container
Isotonic
Nonpyrogenic
Not for injection
Cautions
DO NOT USE FOR ELECTROSURGERY EXCEPT WITH A BIPOLER ELECTRODE
- Do not use unless solution is clear and container is undamaged
- Discard unused content of opened container
- Do not heat this container above 65°Cfre Chlorure de sodium à 0,9% pour irrigation
ger 0,9% Natriumchlorid zum Spülen
ita Sodio cloruro 0.9% per irrigazione
Formula
Natril Chloridum 9 g – Aqua ad injectabilia ad 1000 ml
mOsm/l 308Do Not Re-Use Symbol
Consult Instructions for Use Symbol
European Conformity Symbol
Temperature Limit 25°C Symbol
Sterile SymbolManufacturer Symbol
Baxter Healthcare SA
8010 Zurich, SwitzerlandMade in UK
TH-35-02-060Lot Symbol
Use-by Date Symbol2500
2000
1500
1000
500
-
INGREDIENTS AND APPEARANCE
SODIUM CHLORIDE
sodium chloride irrigantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0338-0110 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 900 mg in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-0110-04 4 in 1 CARTON 11/13/2024 1 NDC:0338-0110-01 3000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 11/13/2024 Labeler - Baxter Healthcare Company (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Ltd 221478644 ANALYSIS(0338-0110) , LABEL(0338-0110) , MANUFACTURE(0338-0110) , PACK(0338-0110) , STERILIZE(0338-0110)