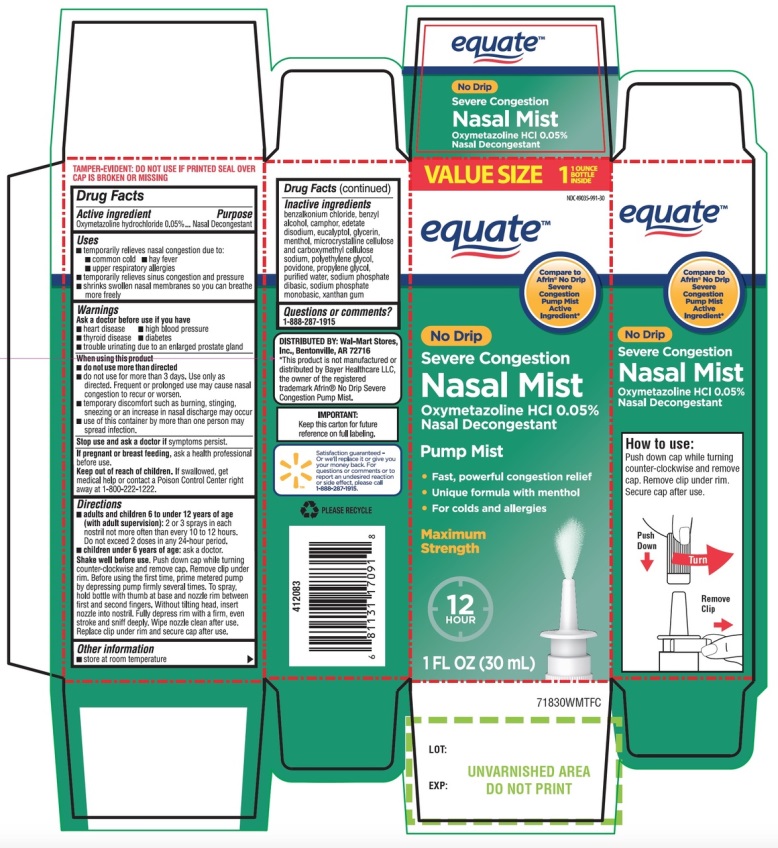
Label: NO DRIP NASAL MIST NASAL DECONGESTANT SEVERE CONGESTION- oxymetazoline hydrochloride spray
- NDC Code(s): 49035-991-30
- Packager: Wal-Mart Stores,Inc.,
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
When using this product
- •
- do not use more than directed
- •
- do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- •
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- •
- use of this container by more than one person may spread infection
-
Directions
- •
- adults and children 6 to under 12 years of age (with adult supervision): 2 or 3 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- •
- children under 6 years of age: ask a doctor
Shake well before use. Push down cap while turning counter-clockwise and remove cap. Remove clip under rim. Before using the first time, prime metered pump by depressing pump firmly several times. To spray, hold bottle with thumb at base and nozzle rim between first and second fingers. Without tilting head, insert nozzle into nostril. Fully depress rim with a firm, even stroke and sniff deeply. Wipe nozzle clean after use. Replace clip under rim and secure cap after use.
- Other information
- Inactive ingredients
-
Principal Display Panel
NDC 49035-991-30
Compare to the active ingredient in Afrin® No-Drip Severe Congestion Pump Mist*
NO DRIP
NASAL MIST
Nasal Decongestant
Oxymetazoline HCl 0.05%
SEVERE CONGESTION
12 Hour Pump Mist
Fast, Powerful Congestion Relief
For Colds & Allergies
Maximum Strength
Unique Formula with Menthol
1 FL OZ (30 mL)
*This product is not manufactured and distributed by Bayer Healthcare LLC, distributor of Afrin® No Drip Severe Congestion Pump Mist.
IMPORTANT: Keep this carton for future reference on full labeling.
Satisfaction guaranteed or we’ll replace it or give you your money back. For questions or comments or to report an undesired reaction or side effect, please call 1-888-287-1915.
TAMPER EVIDENT: DO NOT USE IF SEAL OVER CAP IS BROKEN OR MISSING
DISTRIBUTED BY: Wal-Mart Stores, Inc.,
Bentonville, AR 72716
-
INGREDIENTS AND APPEARANCE
NO DRIP NASAL MIST NASAL DECONGESTANT SEVERE CONGESTION
oxymetazoline hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49035-991 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE HYDROCHLORIDE (UNII: K89MJ0S5VY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE HYDROCHLORIDE 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BENZYL ALCOHOL (UNII: LKG8494WBH) CAMPHOR, (-)- (UNII: 213N3S8275) EDETATE DISODIUM (UNII: 7FLD91C86K) EUCALYPTOL (UNII: RV6J6604TK) GLYCERIN (UNII: PDC6A3C0OX) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE K90 (UNII: RDH86HJV5Z) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49035-991-30 1 in 1 CARTON 08/21/2017 1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 08/21/2017 Labeler - Wal-Mart Stores,Inc., (051957769)