Label: COZAAR- losartan potassium tablet, film coated
- NDC Code(s): 78206-121-01, 78206-122-01, 78206-122-02, 78206-123-01, view more
- Packager: Organon LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use COZAAR safely and effectively. See full prescribing information for COZAAR. COZAAR® (losartan potassium) tablets, for oral ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: FETAL TOXICITY
When pregnancy is detected, discontinue COZAAR as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGE1.1 Hypertension - COZAAR® is indicated for the treatment of hypertension in adults and pediatric patients 6 years of age and older, to lower blood pressure. Lowering blood pressure lowers the ...
-
2 DOSAGE AND ADMINISTRATION2.1 Hypertension - Adult Hypertension - The usual starting dose of COZAAR is 50 mg once daily. The dosage can be increased to a maximum dose of 100 mg once daily as needed to control blood ...
-
3 DOSAGE FORMS AND STRENGTHSCOZAAR, 25 mg, are white, oval, film-coated tablets with code 951 on one side. COZAAR, 50 mg, are white, oval, film-coated tablets with code 952 on one side and scored on the other. COZAAR, 100 ...
-
4 CONTRAINDICATIONSCOZAAR is contraindicated: In patients who are hypersensitive to any component of this product. For coadministration with aliskiren in patients with diabetes.
-
5 WARNINGS AND PRECAUTIONS5.1 Fetal Toxicity - COZAAR can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Agents Increasing Serum Potassium - Coadministration of losartan with other drugs that raise serum potassium levels may result in hyperkalemia. Monitor serum potassium in such ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - COZAAR can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters ...
-
10 OVERDOSAGESignificant lethality was observed in mice and rats after oral administration of 1000 mg/kg and 2000 mg/kg, respectively, about 44 and 170 times the maximum recommended human dose on a mg/m2 ...
-
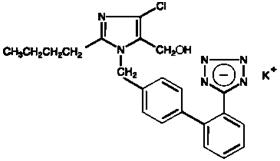
11 DESCRIPTIONCOZAAR (losartan potassium) is an angiotensin II receptor blocker acting on the AT1 receptor subtype. Losartan potassium, a non-peptide molecule, is chemically described as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Angiotensin II [formed from angiotensin I in a reaction catalyzed by angiotensin converting enzyme (ACE, kininase II)] is a potent vasoconstrictor, the primary ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Losartan potassium was not carcinogenic when administered at maximally tolerated dosages to rats and mice for 105 and 92 weeks ...
-
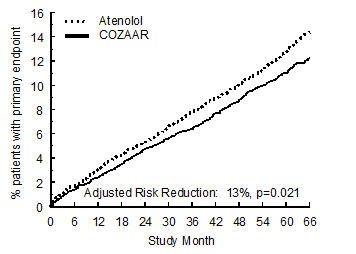
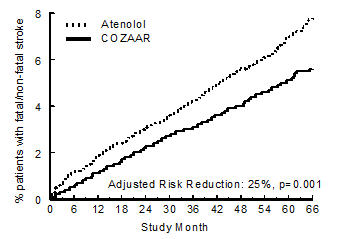
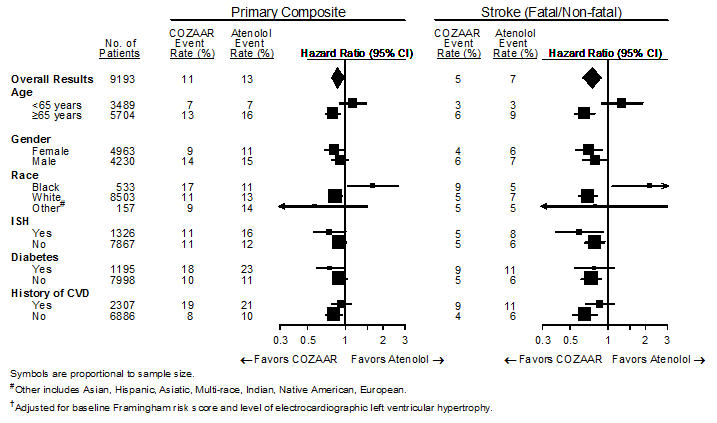
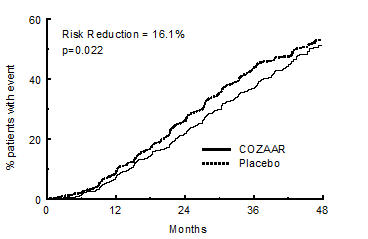
14 CLINICAL STUDIES14.1 Hypertension - Adult Hypertension - The antihypertensive effects of COZAAR were demonstrated principally in 4 placebo-controlled, 6- to 12-week trials of dosages from 10 to 150 mg per day ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGCOZAAR is a white film-coated tablet supplied as follows: LosartanShapeEngraving (reverse)NDC 78206-xxx-xx - Bottle/30Bottle/90 - 25 mgoval951n/a121-01 - 50 mgoval952 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Pregnancy - Advise female patients of childbearing age about the consequences of exposure to COZAAR during ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Organon LLC, a subsidiary of - ORGANON & Co., Jersey City, NJ 07302, USA - For patent information: www.organon.com/our-solutions/patent/ The trademarks depicted herein are owned by ...
-
Patient InformationCOZAAR® (CO-zar)(losartan potassium tablets)25 mg, 50 mg, 100 mgRx onlyRead the Patient Information that comes with COZAAR® before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Organon LLC, a subsidiary of - ORGANON & Co., Jersey City, NJ 07302, USA - For patent information: www.organon.com/our-solutions/patent/ Copyright © 2021 Organon Global Inc. All ...
-
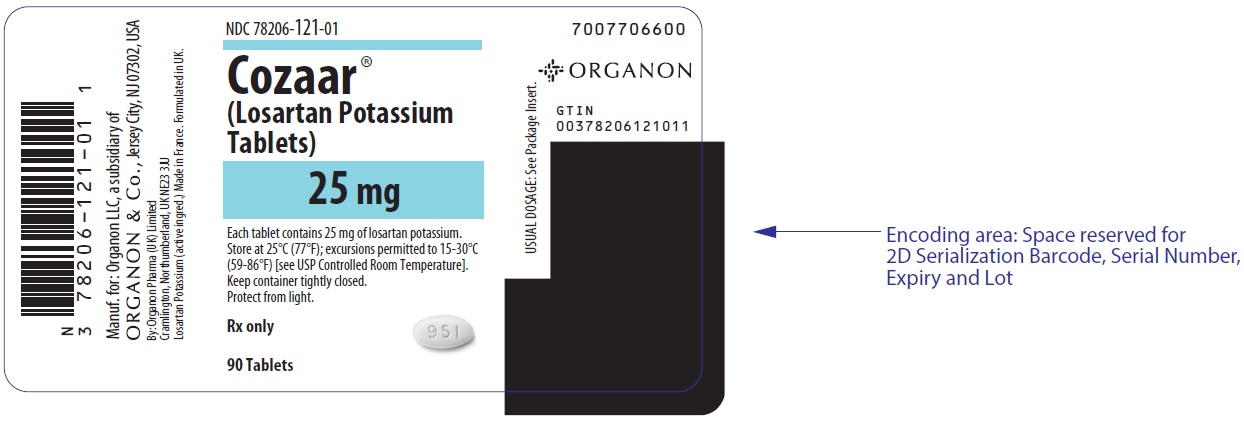
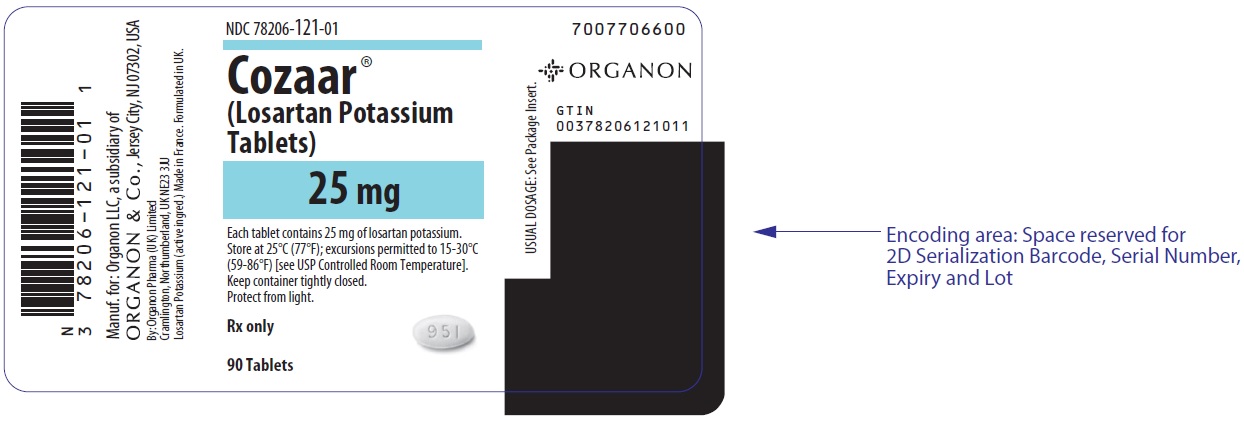
PRINCIPAL DISPLAY PANEL - 25 mg Tablet Bottle LabelNDC 78206-121-01 - Cozaar® (Losartan Potassium - Tablets) 25 mg - Each tablet contains 25 mg of losartan potassium. Store at 25°C (77°F); excursions permitted to 15-30°C - (59-86°F) [see USP ...
-
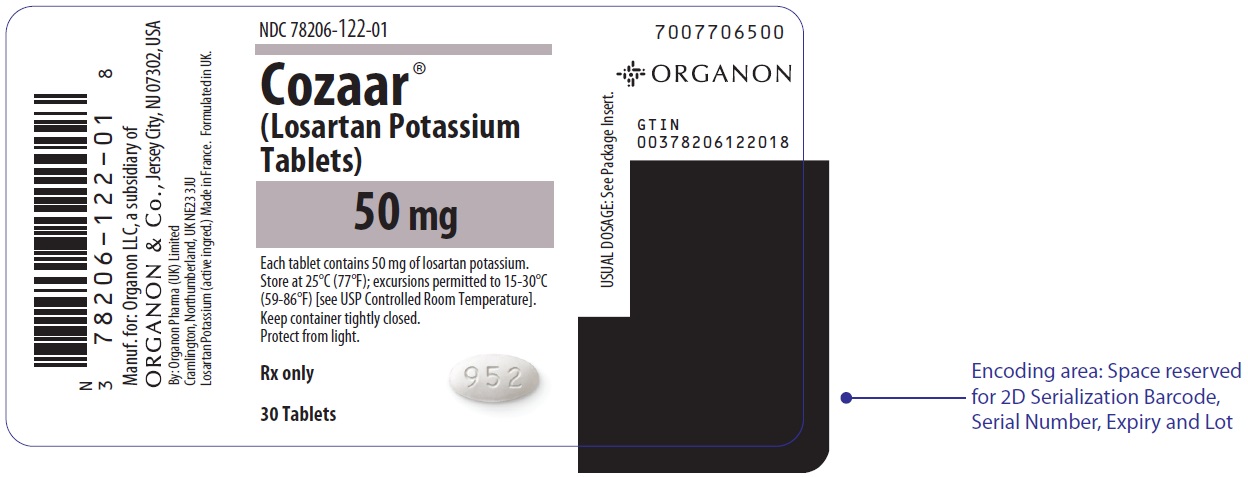
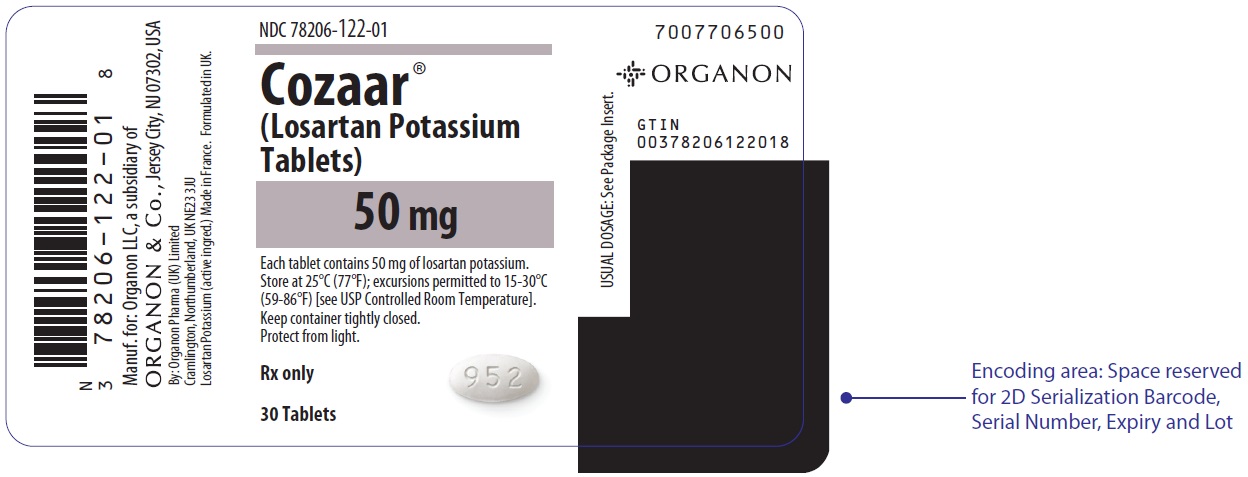
PRINCIPAL DISPLAY PANEL - 50 mg Tablet Bottle LabelNDC 78206-122-01 - Cozaar® (Losartan Potassium - Tablets) 50 mg - Each tablet contains 50 mg of losartan potassium. Store at 25°C (77°F); excursions permitted to 15-30°C - (59-86°F) [see USP ...
-
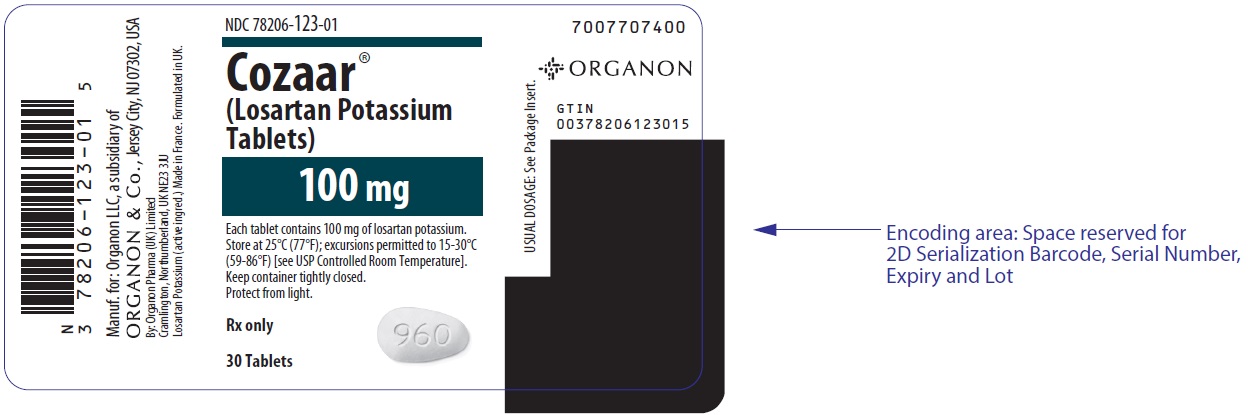
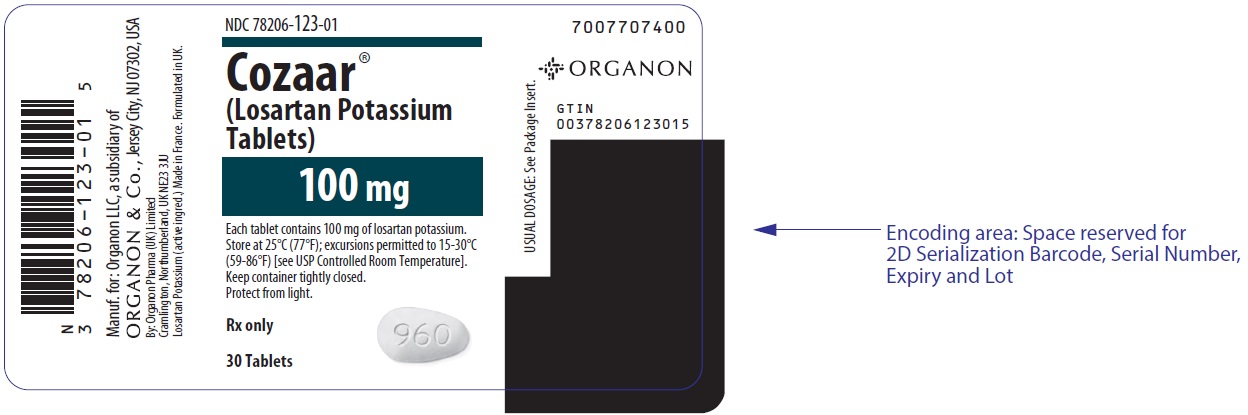
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle LabelNDC 78206-123-01 - Cozaar® (Losartan Potassium - Tablets) 100 mg - Each tablet contains 100 mg of losartan potassium. Store at 25°C (77°F); excursions permitted to 15-30°C - (59-86°F) [see USP ...
-
INGREDIENTS AND APPEARANCEProduct Information