Label: FLUVOXAMINE MALEATE capsule, extended release
- NDC Code(s): 69452-182-13, 69452-182-32, 69452-183-13, 69452-183-32
- Packager: Bionpharma Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FLUVOXAMINE MALEATE EXTENDED-RELEASE CAPSULES safely and effectively. See full prescribing information for FLUVOXAMINE MALEATE EXTENDED-RELEASE CAPSULES.
FLUVOXAMINE MALEATE extended-release capsules, for oral use
Initial U.S. Approval: 2008WARNING: SUICIDALITY and ANTIDEPRESSANTS
See full prescribing information for complete boxed warning.
Increased risk of suicidal thinking and behavior in children, adolescents, and young adults taking antidepressants for major depressive disorder and other psychiatric disorders ( 5.1) .
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Fluvoxamine maleate extended-release capsules are selective serotonin reuptake inhibitor (SSRI) indicated for the treatment of obsessive compulsive disorder (OCD) ( 1). Efficacy was demonstrated in:
- One 12-week study with fluvoxamine maleate extended-release capsules in adults ( 14.1) .
- Two 10-week studies with immediate-release (IR) fluvoxamine tablets in adults and one 10-week study with IR fluvoxamine tablets in children and adolescents ( 14.1, 14.3) .
- One maintenance study with IR fluvoxamine tablets ( 14.2) .
DOSAGE AND ADMINISTRATION
- Adults: Recommended starting dose is 100 mg at bedtime, with weekly increases of 50 mg as tolerated to maximum effect, not to exceed 300 mg/day ( 2.1) .
- Pediatric patients naïve to fluvoxamine maleate: The lowest available dose of fluvoxamine maleate extended-release capsules may not be appropriate ( 2.2) .
- Hepatically impaired: Decreased clearance may require modified dose and titration ( 2.3) .
- Extended treatment: Adjust dose to maintain lowest effective dose; reassess patients periodically ( 2.4) .
- Discontinuation: Gradual dose reduction is recommended ( 2.7, 5.10) .
DOSAGE FORMS AND STRENGTHS
- 100 mg and 150 mg Extended-Release Capsules ( 3)
CONTRAINDICATIONS
- Co-administration of thioridazine, tizanidine, pimozide, alosetron, or ramelteon ( 4) .
- Serotonin Syndrome and MAOIs: Do not use MAOIs intended to treat psychiatric disorders with fluvoxamine maleate extended-release capsules or within 14 days of stopping treatment with fluvoxamine maleate extended-release capsules. Do not use fluvoxamine maleate extended-release capsules within 14 days of stopping an MAOI intended to treat psychiatric disorders. In addition, do not start fluvoxamine maleate extended-release capsules in a patient who is being treated with linezolid or intravenous methylene blue ( 4.1) .
WARNINGS AND PRECAUTIONS
- Clinical Worsening/Suicide Risk:Monitor for clinical worsening of suicidal thoughts/behaviors especially during the initial months of therapy and at times of dose changes ( 5.1) .
- Bipolar Disorder:Screen for bipolar disorder ( 5.1) .
- Serotonin Syndrome:Serotonin syndrome has been reported with SSRIs and SNRIs, including fluvoxamine maleate extended-release capsules, both when taken alone, but especially when co-administered with other serotonergic agents. If such symptoms occur, discontinue fluvoxamine maleate extended-release capsules and serotonergic agents and initiate supportive treatment. If concomitant use of fluvoxamine maleate extended-release capsules with other serotonergic drugs is clinically warranted, patients should be made aware of a potential increased risk for serotonin syndrome, particularly during treatment initiation and dose increases ( 5.2) .
- Angle Closure Glaucoma:Angle closure glaucoma has occurred in patients with untreated anatomically narrow angles treated with antidepressants ( 5.3).
- Other Potentially Important Drug Interactions:Benzodiazepines:Use with caution. Co-administration with diazepam is generally not advisable ( 5.9) . Clozapine:Clozapine levels may be increased and produce orthostatic hypotension or seizures ( 5.9) . Methadone:Co-administration may produce opioid intoxication. Discontinuation of fluvoxamine may produce opioid withdrawal ( 5.9) . Mexiletine:Monitor serum mexiletine levels ( 5.9) . Theophylline:Clearance decreased; reduce theophylline dose by one-third ( 5.9) . Warfarin:Plasma concentrations increased and prothrombin times prolonged; monitor prothrombin time and adjust warfarin dose accordingly ( 5.9) .
- Discontinuation:Symptoms associated with discontinuation have been reported ( 5.10) . In the absence of an emergency, abrupt discontinuation not recommended ( 2.7, 5.2) .
- Abnormal Bleeding:May increase bleeding risk, especially when used with NSAIDs, aspirin, warfarin, or other drugs that affect coagulation ( 5.11) .
- Activation of Mania/Hypomaniahas occurred ( 5.12) .
- Seizures:Avoid administering fluvoxamine in patients with unstable epilepsy; monitor patients with controlled epilepsy; discontinue treatment if seizures occur or frequency increases ( 5.13) .
- Hyponatremia:May occur with SSRIs and SNRIs, including fluvoxamine maleate extended-release capsules. The elderly may be at increased risk. Consider discontinuing in patients with symptomatic hyponatremia ( 5.14) .
- Concomitant Illness:Use caution in patients with diseases or conditions that affect hemodynamic responses or metabolism. Patients with impaired liver function may require a lower starting dose and slower titration ( 5.15) .
- Sexual Dysfunction:Fluvoxamine maleate extended-release capsules may cause symptoms of sexual dysfunction ( 5.17).
ADVERSE REACTIONS
Most common reactions in controlled trials with OCD patients and patients from another studied population (incidence ≥ 5% and at least twice that for placebo) were abnormal ejaculation, anorexia, anorgasmia, asthenia, diarrhea, nausea, somnolence, sweatingand tremor ( 6.2) . The following additional reactions occurred: anxiety, decreased libido, myalgia, pharyngitis,and vomitingin the OCD population; and dyspepsia, dizziness, insomnia,and yawningin another studied population.
To report SUSPECTED ADVERSE REACTIONS, contact Bionpharma Inc., at 1-888-235-BION or 1-888-235-2466 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Drug Interactions (not described in Contraindications or Warnings and Precautions) include the following:
Drugs Inhibiting or Metabolized by Cytochrome P450:Fluvoxamine inhibits several cytochrome P450 isoenzymes (CYP1A2, CYP2C9, CYP3A4, and CYP2C19) ( 7.1) . Carbamazepine:Elevated carbamazepine levels and symptoms of toxicity with co-administration ( 7.2) . Sumatriptan:Rare postmarketing reports of weakness, hyperreflexia, and incoordination following use of an SSRI and sumatriptan. Monitor appropriately if concomitant treatment is clinically warranted ( 7.2) . Tacrine:Co-administration increased tacrine C maxand AUC five- and eight-fold and caused nausea, vomiting, sweating, and diarrhea ( 7.2) . Tricyclic Antidepressants (TCAs):Co-administration significantly increased plasma TCA levels. Use caution; monitor plasma TCA levels; reduce TCA dose if indicated ( 7.2) . Tryptophan:Severe vomiting with co-administration ( 7.2) . Diltiazem:Bradycardia with co-administration ( 7.3) . Propranolol or Metoprolol:Reduce dose if co-administered with fluvoxamine and titrate more cautiously ( 7.3) .
USE IN SPECIFIC POPULATIONS
Specific populations not discussed in Dosage and Administrationor Warnings and Precautionsinclude:
- Pregnancy: Consider both potential risks and benefits when treating a pregnant woman. Infants exposed to SSRIs in pregnancy have developed various complications and may be at risk for persistent pulmonary hypertension of the newborn (PPHN) ( 2.7, 8.1) .
- Nursing mothers: Fluvoxamine is secreted in human breast milk ( 8.3) .
- Geriatric: Use of a lower starting dose may be warranted. Titrate slowly during initiation of therapy ( 2.3, 8.5).
- Smokers: Smokers had a 25% increase in fluvoxamine metabolism ( 7.4) .
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
Suicidality and Antidepressant Drugs
1 INDICATIONS AND USAGE
1.1 Obsessive Compulsive Disorder
2 DOSAGE AND ADMINISTRATION
2.1 OCD (Obsessive Compulsive Disorder)
2.2 Pediatric Patients Naïve to Fluvoxamine Maleate
2.3 Dosage for Elderly or Hepatically Impaired Patients
2.4 Maintenance/Continuation of Extended Treatment
2.5 Switching a Patient to or from a Monoamine Oxidase Inhibitor (MAOI) Intended to Treat Psychiatric Disorders
2.6 Use of Fluvoxamine Maleate Extended-Release Capsules with Other MAOIs such as Linezolid or Methylene Blue
2.7 Discontinuation of Treatment with Fluvoxamine Maleate Extended-Release Capsules
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Monoamine Oxidase Inhibitors (MAOIs)
5 WARNINGS AND PRECAUTIONS
5.1 Clinical Worsening and Suicide Risk
5.2 Serotonin Syndrome
5.3 Angle Closure Glaucoma
5.4 Potential Thioridazine Interaction
5.5 Potential Tizanidine Interaction
5.6 Potential Pimozide Interaction
5.7 Potential Alosetron Interaction
5.8 Potential Ramelteon Interaction
5.9 Other Potentially Important Drug Interactions
5.10 Discontinuation of Treatment
5.11 Abnormal Bleeding
5.12 Activation of Mania/Hypomania
5.13 Seizures
5.14 Hyponatremia
5.15 Use in Patients with Concomitant Illness
5.16 Laboratory Tests
5.17 Sexual Dysfunction
6 ADVERSE REACTIONS
6.1 Clinical Trial Data Sources
6.2 Adverse Reactions Observed in Controlled Trials
6.3 Other Adverse Reactions in OCD Pediatric Population
6.4 Male and Female Sexual Dysfunction with SSRIs
6.5 Weight and Vital Sign Changes
6.6 Laboratory Changes
6.7 ECG Changes
6.8 Other Reactions Observed During the Premarketing Evaluation of Fluvoxamine
6.9 Postmarketing Reports
7 DRUG INTERACTIONS
7.1 Potential Interactions with Drugs that Inhibit or are Metabolized by Cytochrome P450 Isoenzymes
7.2 CNS Active Drugs
7.3 Other Drugs
7.4 Effects of Smoking on Fluvoxamine Metabolism
7.5 Electroconvulsive Therapy (ECT)
7.6 Monoamine Oxidase Inhibitors (MAOIs)
7.7 Serotonergic Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance Class
9.2 Physical and Psychological Dependence
10 OVERDOSAGE
10.1 Human Experience
10.2 Management of Overdosage
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Obsessive Compulsive Disorder (OCD)
14.2 Adult OCD Maintenance Study with Immediate-Release Fluvoxamine Maleate Tablets
14.3 Pediatric OCD Study
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
17.1 Clinical Worsening and Suicide Risk
17.2 Serotonin Syndrome
17.3 Contraindicated Medications
17.4 Other Potentially Hazardous Drug Interactions
17.5 Abnormal Bleeding
17.6 Angle Closure Glaucoma
17.7 Interference with Cognitive or Motor Performance
17.8 Pregnancy
17.9 Nursing
17.10 Alcohol
17.11 Allergic Reactions
17.12 Sexual Dysfunction
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of fluvoxamine maleate extended-release capsules or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber [see Warnings and Precautions-Clinical Worsening and Suicide Risk ( 5.1) and Use in Specific Populations-Pediatric Use ( 8.4)] .
-
1 INDICATIONS AND USAGE
1.1 Obsessive Compulsive Disorder
Fluvoxamine maleate extended-release capsules are indicated for the treatment of obsessive compulsive disorder (OCD), as defined in the DSM-IV. Obsessive compulsive disorder is characterized by recurrent and persistent ideas, thoughts, impulses, or images (obsessions) that are ego-dystonic and/or repetitive, purposeful, and intentional behaviors (compulsions) that are recognized by the person as excessive or unreasonable. The obsessions or compulsions cause marked distress, are time-consuming, or significantly interfere with social or occupational functioning.
The efficacy of fluvoxamine maleate extended-release capsules was demonstrated in one 12-week trial in adults with fluvoxamine maleate extended-release capsules as well as in two 10-week trials in adults and in one 10-week trial in children and adolescents (ages 8 to 17 years) with immediate-release fluvoxamine tablets in outpatients with the diagnosis of OCD as defined in DSM-IV or DSM-III-R [see Clinical Studies (14.1, 14.3)] .
The efficacy of fluvoxamine for long-term use was established in one maintenance study in adults with immediate-release fluvoxamine tablets [see Clinical Studies (14.2)] . The health care provider who elects to prescribe fluvoxamine maleate extended-release capsules for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient [see Dosage and Administration (2.4) ].
-
2 DOSAGE AND ADMINISTRATION
2.1 OCD (Obsessive Compulsive Disorder)
The recommended starting dose is 100 mg at bedtime, with weekly increases of 50 mg as tolerated to maximum therapeutic benefit, not to exceed 300 mg per day.
Capsules should not be crushed or chewed.
2.2 Pediatric Patients Naïve to Fluvoxamine Maleate
Physicians should consider that the lowest available dose of fluvoxamine maleate extended-release capsules may not be appropriate for pediatric patients naïve to fluvoxamine maleate.
2.3 Dosage for Elderly or Hepatically Impaired Patients
Elderly patients and those with hepatic impairment have been observed to have a decreased clearance of fluvoxamine maleate. Consequently, it may be appropriate to titrate slowly following the initial dose of 100 mg in these patient groups.
2.4 Maintenance/Continuation of Extended Treatment
Although the efficacy of fluvoxamine maleate extended-release capsules beyond 12 weeks of dosing has not been documented in controlled trials, OCD is a chronic condition, and it is reasonable to consider continuation for a responding patient. The benefit of maintaining patients with OCD on immediate-release fluvoxamine maleate tablets after achieving a response for an average duration of about 4 weeks in a 10-week single-blind phase during which patients were titrated to effect was demonstrated in a controlled trial [see Clinical Studies( 14.2)] . Dosage adjustments should be made to maintain the patient on the lowest effective dosage, and patients should be periodically reassessed to determine the need for continued treatment.
2.5 Switching a Patient to or from a Monoamine Oxidase Inhibitor (MAOI) Intended to Treat Psychiatric Disorders
At least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with fluvoxamine maleate extended-release capsules. Conversely, at least 14 days should be allowed after stopping fluvoxamine maleate extended-release capsules before starting an MAOI intended to treat psychiatric disorders [see Contraindications (4.1)] .
2.6 Use of Fluvoxamine Maleate Extended-Release Capsules with Other MAOIs such as Linezolid or Methylene Blue
Do not start fluvoxamine maleate extended-release capsules in a patient who is being treated with linezolid or intravenous methylene blue because there is an increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, other interventions, including hospitalization, should be considered [see Contraindications (4.1)] .
In some cases, a patient already receiving fluvoxamine maleate extended-release capsules therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, fluvoxamine maleate extended-release capsules should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for two weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with fluvoxamine maleate extended-release capsules may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue [see Warnings and Precautions (5.2)] .
The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with fluvoxamine maleate extended-release capsules is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use [see Warnings and Precautions (5.2)] .
2.7 Discontinuation of Treatment with Fluvoxamine Maleate Extended-Release Capsules
Symptoms associated with discontinuation of other SSRIs or SNRIs have been reported [see Warnings and Precautions (5.10)] . Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the health care provider may continue decreasing the dose but at a more gradual rate.
-
3 DOSAGE FORMS AND STRENGTHS
Fluvoxamine maleate extended-release capsules are available as:
100 mg extended-release capsules: white to off-white spherical pellets filled in size “2” hard gelatin capsule with purple opaque cap imprinted with “FL” in black ink and white opaque body imprinted with “100” in black ink.
150 mg extended-release capsules: white to off-white spherical pellets filled in size “1” hard gelatin capsule with purple opaque cap imprinted with “FL” in black ink and white opaque body imprinted with “150” in black ink.
-
4 CONTRAINDICATIONS
Co-administration of thioridazine, tizanidine, pimozide, alosetron, or ramelteon with fluvoxamine maleate extended-release capsules is contraindicated [see Warnings and Precautions (5.4 to 5.8)] .
4.1 Monoamine Oxidase Inhibitors (MAOIs)
The use of MAOIs intended to treat psychiatric disorders with fluvoxamine maleate extended‑release capsules or within 14 days of stopping treatment with fluvoxamine maleate extended-release capsules is contraindicated because of an increased risk of serotonin syndrome. The use of fluvoxamine maleate extended-release capsules within 14 days of stopping an MAOI intended to treat psychiatric disorders is also contraindicated [see Dosage and Administration ( 2.5) and Warnings and Precautions ( 5.2)] .
Starting fluvoxamine maleate extended-release capsules in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue is also contraindicated because of an increased risk of serotonin syndrome [see Dosage and Administration ( 2.6) and Warnings and Precautions ( 5.2)] .
-
5 WARNINGS AND PRECAUTIONS
5.1 Clinical Worsening and Suicide Risk
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. The pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults age 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4,400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1,000 patients treated) are provided in Table 1.
TABLE 1 DRUG-PLACEBO DIFFERENCES IN NUMBER OF CASES OF SUICIDALITY PER 1,000 PATIENTS TREATED AGE RANGE DRUG-RELATED INCREASES < 18 14 ADDITIONAL CASES 18 to 24 5 ADDITIONAL CASES AGE RANGE DRUG-RELATED DECREASES 25 to 64 1 FEWER CASE ≥ 65 6 FEWER CASES No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about the drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient’s presenting symptoms.
If the decision has been made to discontinue treatment, medication should be tapered, as rapidly as is feasible, but with recognition that abrupt discontinuation can be associated with certain symptoms [see Dosage and Administration–Discontinuation of Treatment with Fluvoxamine Maleate Extended-Release Capsules (2.7), for a description of the risks of discontinuation of fluvoxamine maleate extended-release capsules ].
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers.Prescriptions for fluvoxamine maleate extended-release capsules should be written for the smallest quantity of capsules consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder:A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that fluvoxamine maleate extended-release capsules are not approved for use in treating bipolar depression.
5.2 Serotonin Syndrome
The development of a potentially life-threatening serotonin syndrome has been reported with SNRIs and SSRIs, including fluvoxamine maleate extended-release capsules, alone but particularly with concomitant use of serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, meperidine, methadone, lithium, tramadol, tryptophan, buspirone, amphetamines, and St. John’s Wort) and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue).
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotonin syndrome.
The concomitant use of fluvoxamine maleate extended-release capsules with MAOIs intended to treat psychiatric disorders is contraindicated. Fluvoxamine maleate extended-release capsules should also not be started in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue. All reports with methylene blue that provided information on the route of administration involved intravenous administration in the dose range of 1 mg/kg to 8 mg/kg. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection) or at lower doses. There may be circumstances when it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking fluvoxamine maleate extended-release capsules. Fluvoxamine maleate extended-release capsules should be discontinued before initiating treatment with the MAOI [see Contraindications (4.1) and Dosage and Administration (2.5, 2.6) ].
If concomitant use of fluvoxamine maleate extended-release capsules with other serotonergic drugs, including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, amphetamines, and St. John's Wort, is clinically warranted, patients should be made aware of a potential increased risk for serotonin syndrome, particularly during treatment initiation and dose increases.
Treatment with fluvoxamine maleate extended-release capsules and any concomitant serotonergic agents should be discontinued immediately if the above events occur, and supportive symptomatic treatment should be initiated.
5.3 Angle Closure Glaucoma
Angle Closure Glaucoma: The pupillary dilation that occurs following use of many antidepressant drugs including fluvoxamine maleate extended-release capsules may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy.
5.4 Potential Thioridazine Interaction
The effect of fluvoxamine (25 mg immediate-release tablets given twice daily for one week) on thioridazine steady-state concentrations was evaluated in 10 male inpatients with schizophrenia. Concentrations of thioridazine and its two active metabolites, mesoridazine and sulforidazine, increased 3-fold following co-administration of fluvoxamine.
Thioridazine administration produces a dose-related prolongation of the QTc interval, which is associated with serious ventricular arrhythmias, such as torsades de pointes-type arrhythmias, and sudden death. It is likely that this experience underestimates the degree of risk that might occur with higher doses of thioridazine. Moreover, the effect of fluvoxamine may be even more pronounced when it is administered at higher doses.
Therefore, fluvoxamine maleate extended-release capsules should not be co-administered with thioridazine [see Contraindications (4)] .
5.5 Potential Tizanidine Interaction
Fluvoxamine is a potent inhibitor of CYP1A2, and tizanidine is a CYP1A2 substrate. The effect of immediate-release fluvoxamine maleate tablets (100 mg daily for four days) on the pharmacokinetics and pharmacodynamics of a single dose of tizanidine has been studied in 10 healthy male subjects. Tizanidine C maxwas increased approximately 12-fold (range 5-fold to 32-fold), elimination half-life was increased by almost 3-fold, and AUC increased 33-fold (range 14-fold to 103-fold). The mean maximal effect on blood pressure was a 35 mm Hg decrease in systolic blood pressure, a 20 mm Hg decrease in diastolic blood pressure, and a 4 beat/min decrease in heart rate. Drowsiness was significantly increased, and performance on the psychomotor task was significantly impaired. Fluvoxamine maleate extended-release capsules and tizanidine should not be used together [see Contraindications (4)] .
5.6 Potential Pimozide Interaction
Pimozide is metabolized by the CYP3A4 isozyme and it has been demonstrated that ketoconazole, a potent inhibitor of CYP3A4, blocks the metabolism of this drug, resulting in increased plasma concentrations of parent drug. An increased plasma concentration of pimozide causes QT prolongation and has been associated with torsades de pointes-type ventricular tachycardia, sometimes fatal. As noted below, a substantial pharmacokinetic interaction has been observed for fluvoxamine in combination with alprazolam, a drug that is known to be metabolized by the CYP3A4 isozyme. Although it has not been definitively demonstrated that fluvoxamine is a potent CYP3A4 inhibitor, it is likely to be, given the substantial interaction of fluvoxamine with alprazolam. Consequently, it is recommended that fluvoxamine not be used in combination with pimozide [see Contraindications (4)] .
5.7 Potential Alosetron Interaction
In a pharmacokinetic study, 40 healthy female subjects received fluvoxamine in escalating doses from 50 to 200 mg/day for 16 days with co-administration of alosetron 1 mg on the last day. Fluvoxamine increased mean alosetron plasma concentrations (AUC) approximately 6-fold and prolonged the half-life by approximately 3-fold. Consequently, it is recommended that fluvoxamine maleate extended-release capsules not be used in combination with alosetron [see Contraindications (4) and Lotronex ®(alosetron) package insert ].
5.8 Potential Ramelteon Interaction
When immediate-release fluvoxamine maleate tablets 100 mg twice daily were administered for 3 days prior to single-dose co-administration of ramelteon 16 mg and immediate-release fluvoxamine maleate tablets, the AUC for ramelteon increased approximately 190-fold and the C maxincreased approximately 70-fold compared to ramelteon administered alone. Ramelteon should not be used in combination with fluvoxamine maleate extended-release capsules [see Contraindications (4)] .
5.9 Other Potentially Important Drug Interactions
Benzodiazepines:Benzodiazepines metabolized by hepatic oxidation (e.g., alprazolam, midazolam, triazolam, etc.) should be used with caution because the clearance of these drugs is likely to be reduced by fluvoxamine. The clearance of benzodiazepines metabolized by glucuronidation (e.g., lorazepam, oxazepam, temazepam) is unlikely to be affected by fluvoxamine.
Alprazolam- When immediate-release fluvoxamine maleate tablets (100 mg given once daily) and alprazolam (1 mg given 4 times per day) were co-administered to steady state, plasma concentrations and other pharmacokinetic parameters (AUC, C max, T ½) of alprazolam were approximately twice those observed when alprazolam was administered alone; oral clearance was reduced by about 50%. The elevated plasma alprazolam concentrations resulted in decreased psychomotor performance and memory. This interaction, which has not been investigated using higher doses of fluvoxamine, may be more pronounced if a 300 mg daily dose is co-administered, particularly since fluvoxamine exhibits non-linear pharmacokinetics over the dosage range 100 to 300 mg. If alprazolam is co-administered with fluvoxamine maleate extended-release capsules, the initial alprazolam dosage should be at least halved and titration to the lowest effective dose is recommended. No dosage adjustment is required for fluvoxamine maleate extended-release capsules.
Diazepam- The co-administration of fluvoxamine maleate extended-release capsules and diazepam is generally not advisable. Because fluvoxamine reduces the clearance of both diazepam and its active metabolite, N-desmethyldiazepam, there is a strong likelihood of substantial accumulation of both species during chronic co-administration.
Evidence supporting the conclusion that it is inadvisable to co-administer fluvoxamine and diazepam is derived from a study in which healthy volunteers taking 150 mg/day of immediate-release fluvoxamine maleate tablets were administered a single oral dose of 10 mg of diazepam. In these subjects (N = 8), the clearance of diazepam was reduced by 65% and that of N-desmethyldiazepam to a level that was too low to measure over the course of the 2-week-long study.
It is likely that this experience significantly underestimates the degree of accumulation that might occur with repeated diazepam administration. Moreover, as noted with alprazolam, the effect of fluvoxamine may even be more pronounced when it is administered at higher doses.
Accordingly, diazepam and fluvoxamine should not ordinarily be co-administered.
Clozapine:Elevated serum levels of clozapine have been reported in patients taking immediate-release fluvoxamine maleate tablets and clozapine. Since clozapine-related seizures and orthostatic hypotension appear to be dose related, the risk of these adverse reactions may be higher when fluvoxamine and clozapine are co-administered. Patients should be closely monitored when fluvoxamine maleate extended-release capsules and clozapine are used concurrently.
Methadone:Significantly increased methadone (plasma level:dose) ratios have been reported when immediate-release fluvoxamine maleate tablets were administered to patients receiving maintenance methadone treatment, with symptoms of opioid intoxication in one patient. Opioid withdrawal symptoms were reported following fluvoxamine maleate discontinuation in another patient.
Mexiletine:The effect of steady-state fluvoxamine (50 mg given twice daily for 7 days) on the single dose pharmacokinetics of mexiletine (200 mg) was evaluated in 6 healthy Japanese males. The clearance of mexiletine was reduced by 38% following co-administration with fluvoxamine compared to mexiletine alone. If fluvoxamine and mexiletine are co-administered, serum mexiletine levels should be monitored.
Theophylline:The effect of steady-state immediate-release fluvoxamine maleate tablets (50 mg tablets given twice daily) on the pharmacokinetics of a single dose of theophylline (375 mg as 442 mg aminophylline) was evaluated in 12 healthy non-smoking, male volunteers. The clearance of theophylline was decreased approximately 3-fold. Therefore, if theophylline is co-administered with fluvoxamine maleate, its dose should be reduced to one-third of the usual daily maintenance dose and plasma concentrations of theophylline should be monitored. No dosage adjustment is required for fluvoxamine maleate extended-release capsules.
Warfarin and Other Drugs that Interfere with Hemostasis (NSAIDs, Aspirin, etc.):Serotonin release by platelets plays an important role in hemostasis. Epidemiological studies of the case-control and cohort design have demonstrated an association between use of psychotropic drugs that interfere with serotonin reuptake and the occurrence of upper gastrointestinal bleeding. These studies have also shown that concurrent use of an NSAID or aspirin may potentiate this risk of bleeding. Thus, patients should be cautioned about the use of such drugs concurrently with fluvoxamine [see Warnings and Precautions-Abnormal Bleeding( 5.11)] .
Warfarin -When immediate-release fluvoxamine maleate tablets (50 mg given 3 times daily) were administered concomitantly with warfarin for two weeks, warfarin plasma concentrations increased by 98% and prothrombin times were prolonged. Thus patients receiving oral anticoagulants and fluvoxamine maleate extended-release capsules should have their prothrombin time monitored and their anticoagulant dose adjusted accordingly. No dosage adjustment is required for fluvoxamine maleate extended-release capsules.
5.10 Discontinuation of Treatment
During marketing of immediate-release fluvoxamine maleate tablets and other SSRIs and SNRIs (serotonin and norepinephrine reuptake inhibitors), there have been spontaneous reports of adverse reactions occurring upon discontinuation of these drugs, particularly when abrupt, including the following: dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesias, such as electric shock sensations), anxiety, confusion, headache, lethargy, emotional lability, insomnia, and hypomania. While these reactions are generally self-limiting, there have been reports of serious discontinuation symptoms.
Patients should be monitored for these symptoms when discontinuing treatment with fluvoxamine maleate extended-release capsules. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the health care provider may continue decreasing the dose but at a more gradual rate [see Dosage and Administration (2.7)] .
5.11 Abnormal Bleeding
SSRIs and SNRIs, including fluvoxamine maleate extended-release capsules, may increase the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs, warfarin, and other anticoagulants may add to this risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding.Based on data from the published observational studies, exposure to SSRIs, particularly in the month before delivery, has been associated with a less than 2-fold increase in the risk of postpartum hemorrhage [see Use in Specific Populations ( 8.1)] . Bleeding events related to SSRIs and SNRIs use have ranged from ecchymoses, hematomas, epistaxis, and petechiae to life-threatening hemorrhages.
Patients should be cautioned about the increased risk of bleeding associated with the concomitant use of fluvoxamine maleate extended-release capsules and NSAIDs, aspirin, or other drugs that affect coagulation [see Warnings and Precautions ( 5.9)].
5.12 Activation of Mania/Hypomania
During premarketing studies of immediate-release fluvoxamine maleate tablets involving primarily depressed patients, hypomania or mania occurred in approximately 1% of patients treated with fluvoxamine. In a 10-week pediatric OCD study, 2 out of 57 patients (4%) treated with fluvoxamine experienced manic reactions, compared to none of 63 placebo patients. Activation of mania/hypomania has also been reported in a small proportion of patients with major affective disorder who were treated with other marketed antidepressants. As with all antidepressants, fluvoxamine maleate extended-release capsules should be used cautiously in patients with a history of mania.
5.13 Seizures
During premarketing studies with immediate-release fluvoxamine maleate tablets, seizures were reported in 0.2% of fluvoxamine-treated patients. Caution is recommended when the drug is administered to patients with a history of convulsive disorders. Fluvoxamine should be avoided in patients with unstable epilepsy, and patients with controlled epilepsy should be carefully monitored. Treatment with fluvoxamine should be discontinued if seizures occur or if seizure frequency increases.
5.14 Hyponatremia
Hyponatremia may occur as a result of treatment with SSRIs and SNRIs, including fluvoxamine maleate extended-release capsules. In many cases, this hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Cases with serum sodium lower than 110 mmol/L have been reported. Elderly patients may be at greater risk of developing hyponatremia with SSRIs and SNRIs [see Use in Specific Populations, Geriatric Use( 8.5)] . Also, patients taking diuretics or who are otherwise volume depleted may be at greater risk. Discontinuation of fluvoxamine maleate extended-release capsules should be considered in patients with symptomatic hyponatremia and appropriate medical intervention should be instituted.
Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which may lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death.
5.15 Use in Patients with Concomitant Illness
Closely monitored clinical experience with fluvoxamine maleate extended-release capsules in patients with concomitant systemic illness is limited. Caution is advised in administering fluvoxamine maleate extended-release capsules to patients with diseases or conditions that could affect hemodynamic responses or metabolism.
Fluvoxamine maleate extended-release capsules or immediate-release fluvoxamine maleate tablets have not been evaluated or used to any appreciable extent in patients with a recent history of myocardial infarction or unstable heart disease. Patients with these diagnoses were systematically excluded from many clinical studies during premarketing testing of these products. Evaluation of the electrocardiograms for patients with depression or OCD who participated in premarketing studies revealed no differences between fluvoxamine and placebo in the emergence of clinically important ECG changes.
Patients with Hepatic Impairment -In patients with liver dysfunction, following administration of immediate-release fluvoxamine maleate tablets, fluvoxamine clearance was decreased by approximately 30%. Patients with liver dysfunction should begin with a low dose of fluvoxamine maleate extended-release capsules and increase it slowly with careful monitoring.
5.17 Sexual Dysfunction
Use of SSRIs, including fluvoxamine maleate extended-release capsules, may cause symptoms of sexual dysfunction [see Adverse Reactions ( 6.1)]. In male patients, SSRI use may result in ejaculatory delay or failure, decreased libido, and erectile dysfunction. In female patients, SSRI use may result in decreased libido and delayed or absent orgasm.
It is important for prescribers to inquire about sexual function prior to initiation of fluvoxamine maleate extended-release capsules and to inquire specifically about changes in sexual function during treatment, because sexual function may not be spontaneously reported. When evaluating changes in sexual function, obtaining a detailed history (including timing of symptom onset) is important because sexual symptoms may have other causes, including the underlying psychiatric disorder. Discuss potential management strategies to support patients in making informed decisions about treatment.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
6.1 Clinical Trial Data Sources
Fluvoxamine maleate extended-release capsules were studied in one 12-week controlled trial in patients with OCD (N = 124; mean exposure 66.6 days) and in two 12-week controlled trials for another condition (N = 279; mean exposure 59.2 days). Patients in these trials were initiated on 100 mg/day and were titrated in 50 mg increments over the first 6 weeks to within a range of 100 mg to 300 mg/day. The reactions listed in Table 2 show reactions from the two populations separately. Table 3 shows reactions from the three controlled studies combined.
6.2 Adverse Reactions Observed in Controlled Trials
Adverse Reactions Associated with Discontinuation of Treatment:Of the 124 patients with OCD and 279 patients in other studies treated with fluvoxamine maleate extended-release capsules in controlled clinical trials, 19% and 26% discontinued treatment due to an adverse reaction. The most common reactions (≥ 1%) associated with discontinuation and considered to be drug related (i.e., those reactions associated with dropout at a rate at least twice that of placebo) were anorexia(including, but not limited to, loss of appetite and decreased appetite) (1%), anxiety (3%), asthenia (3%), diarrhea (2%), dizziness (4%), headache (2%), insomnia (5%), nausea (7%), nervousness (1%), somnolence (5%),and thinking abnormal (1%).
Commonly Observed Adverse Reactions:Fluvoxamine maleate extended-release capsules have been studied in one controlled trial in patients with OCD (N = 124) and two controlled trials for another condition (N = 279). In general, adverse reaction rates were similar in the two data sets as well as in a study of pediatric patients with OCD treated with immediate-release fluvoxamine maleate tablets. The most commonly observed treatment-emergent adverse reactions associated with the use of fluvoxamine maleate extended-release capsules and likely to be drug-related (incidence of 5% or greater and at least twice that for placebo) and derived from Table 2 were: abnormal ejaculation, anorexia, anorgasmia, asthenia, diarrhea, nausea, somnolence, sweating,and tremor. In the one controlled trial in patients with OCD, the following additional reactions occurred at an incidence of 5% or greater and at least twice that for placebo: anxiety, decreased libido, myalgia, pharyngitis,and vomiting. The following additional reactions occurred in another studied population: dyspepsia, dizziness, insomnia,and yawning. In a study evaluating immediate-release fluvoxamine maleate tablets in pediatric patients with OCD, the following additional reactions were identified using the above rule: agitation, depression, dysmenorrhea, flatulence, hyperkinesia,and rash.
Adverse Reactions Occurring at an Incidence of ≥ 2%:Table 2 enumerates adverse reactions that occurred in adults at a frequency of 2% or more, and were more frequent than in the placebo group, among patients treated with fluvoxamine maleate extended-release capsules in two short-term, placebo-controlled trials (12 weeks) in another population and one short-term placebo-controlled OCD trial (12 weeks) and in which patients were dosed once-a-day in a range of 100 to 300 mg/day. This table shows the percentage of patients in each group who had at least one occurrence of a reaction at some time during their treatment. Reported adverse reactions were classified using a COSTART-based Dictionary terminology.
The prescriber should be aware that these figures cannot be used to predict the incidence of side effects in the course of usual medical practice where patient characteristics and other factors may differ from those that prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. The cited figures, however, do provide the prescribing health care provider with some basis for estimating the relative contribution of drug and non-drug factors to the side-effect incidence rate in the population studied.
TABLE 2 TREATMENT-EMERGENT ADVERSE REACTION INCIDENCE RATES BY BODY SYSTEM IN ADULT OCD PATIENTS AND ANOTHER STUDIED POPULATION1 PERCENTAGE OF PATIENTS REPORTING REACTION OBSESSIVE COMPULSIVE DISORDER OTHER STUDIED POPULATION BODY SYSTEM/ADVERSE REACTION FLUVOXAMINE MALEATE FLUVOXAMINE MALEATE EXTENDED-RELEASE CAPSULES PLACEBO EXTENDED-RELEASE CAPSULES PLACEBO N = 124 N = 124 N = 279 N = 276 1Events for which fluvoxamine maleate incidence was equal to or less than placebo include the following for OCD patients: abdominal pain, flu syndrome, infection, palpitation, flatulence, increased appetite, weight gain, abnormal dreams, amnesia, hypertonia, nervousness, paresthesia, increased cough, dyspnea, rhinitis, and ear pain. In the other studied population, the following events were seen: abdominal pain, accidental injury, back pain, flu syndrome, infection, pain, flatulence, pharyngitis, rhinitis, rash, and dysmenorrhea. 2Term includes body aches/pains, dental pain, pain from surgery, unspecified pain, and general pain secondary to injuries (sprains, fractures). 3Includes, but is not limited to, loss of appetite and decreased appetite. BODY AS A WHOLE Headache 32 31 35 30 Asthenia 26 8 24 10 Pain 2 10 8 – – Abdominal Pain – – 5 4 Accidental Injury 5 3 – – Chest Pain – – 3 1 Viral Infection 2 <1 – – CARDIOVASCULAR Palpitation – – 3 1 Vasodilatation – – 2 <1 Hypertension 2 <1 – – DIGESTIVE SYSTEM Nausea 34 13 39 11 Diarrhea 18 8 14 5 Anorexia 3 13 5 14 1 Dyspepsia 8 5 10 4 Constipation 4 <1 6 5 Vomiting 6 2 – – Tooth Disorder 2 <1 – – Liver Function Test Abnormal – – 2 <1 Gingivitis 2 0 – – HEMIC AND LYMPHATIC Ecchymosis 4 2 – – METABOLIC AND NUTRITIONAL DISORDERS Weight Loss 2 <1 – – MUSCULOSKELETAL Myalgia 5 2 – – NERVOUS SYSTEM Insomnia 35 20 32 13 Somnolence 27 11 26 9 Dizziness 12 10 15 7 Dry Mouth 10 9 11 8 Nervousness – – 10 9 Libido Decreased 6 2 6 4 Male 10 5 8 6 Female 4 1 4 3 Anxiety 6 2 8 5 Tremor 6 0 8 <1 Abnormal Thinking 3 <1 3 2 Abnormal Dreams – – 3 2 Agitation 2 <1 3 <1 Hypertonia – – 2 1 Apathy 3 0 – – Paresthesia – – 3 2 Neurosis 2 <1 – – Twitching 2 0 – – RESPIRATORY SYSTEM Pharyngitis 6 <1 – – Yawn 2 0 5 <1 Laryngitis 3 0 – – Bronchitis – – 2 1 Epistaxis 2 0 – – SKIN Sweating 7 <1 6 2 Acne 2 0 – – SPECIAL SENSES Taste Perversion 2 <1 2 <1 Amblyopia 2 <1 – – UROGENITAL Abnormal Ejaculation 10 0 11 2 Anorgasmia 5 0 5 1 Male 4 0 4 2 Female 5 0 5 0 Menorrhagia 3 0 – – Sexual Function Abnormal 2 <1 3 <1 Male 4 3 2 1 Female 0 0 3 0 Urinary Tract Infection – – 2 <1 Polyuria 2 <1 – – 6.3 Other Adverse Reactions in OCD Pediatric Population
In pediatric patients (N = 57) treated with immediate-release fluvoxamine maleate tablets, the overall profile of adverse reactions was generally similar to that seen in adult studies, as shown in Table 2. However, the following adverse reactions, not appearing in Table 2, were reported in two or more of the pediatric patients and were more frequent with immediate-release fluvoxamine maleate tablets than with placebo: cough increase, dysmenorrhea, ecchymosis, emotional lability, epistaxis, hyperkinesia, manic reaction, rash, sinusitis, and weight decrease.
6.4 Male and Female Sexual Dysfunction with SSRIs
Although changes in sexual desire, sexual performance, and sexual satisfaction often occur as manifestations of a psychiatric disorder and with aging, they may also be a consequence of pharmacologic treatment. In particular, some evidence suggests that selective serotonin reuptake inhibitors (SSRIs) can cause such untoward sexual experiences.
Reliable estimates of the incidence and severity of untoward experiences involving sexual desire, performance, and satisfaction are difficult to obtain, however, in part because patients and health care providers may be reluctant to discuss them. Accordingly, estimates of the incidence of untoward sexual experience and performance cited in product labeling are likely to underestimate their actual incidence.
Table 3 displays the incidence of sexual side effects reported by at least 2% of patients taking fluvoxamine maleate extended-release capsules in placebo-controlled trials.
TABLE 3 PERCENTAGE OF PATIENTS REPORTING SEXUAL ADVERSE REACTIONS IN PLACEBO-CONTROLLED TRIALS Fluvoxamine maleate extended-release capsules Placebo N = 403 N = 400 Abnormal Ejaculation 11 2 Anorgasmia Male 4 1 Female 5 0 Impotence 2 2 Libido Decreased Male 8 5 Female 4 2 Sexual Function Abnormal Male 3 1 Female 2 0 Fluvoxamine treatment has been associated with several cases of priapism. In those cases with a known outcome, patients recovered without sequelae and upon discontinuation of fluvoxamine.
While it is difficult to know the precise risk of sexual dysfunction associated with the use of SSRIs, health care providers should routinely inquire about such possible side effects.
6.5 Weight and Vital Sign Changes
No statistically significant differences in weight gain or loss were found between patients treated with fluvoxamine maleate extended-release capsules or placebo. Comparisons of immediate-release fluvoxamine maleate tablets or fluvoxamine maleate extended-release capsules versus placebo groups in separate short-term trials on (1) median change from baseline on various vital signs variables and on (2) incidence of patients meeting criteria for potentially important changes from baseline on various measures of vital signs variables revealed no important differences between fluvoxamine maleate and placebo.
6.6 Laboratory Changes
Comparisons of immediate-release fluvoxamine maleate tablets or fluvoxamine maleate extended-release capsules versus placebo groups in separate short-term trials on (1) median change from baseline on various serum chemistry, hematology, and urinalysis variables and on (2) incidence of patients meeting criteria for potentially important changes from baseline on various serum chemistry, hematology, and urinalysis variables revealed no important differences between fluvoxamine maleate and placebo.
6.7 ECG Changes
Comparisons of immediate-release fluvoxamine maleate tablets or fluvoxamine maleate extended-release capsules and placebo groups in separate pools of short-term OCD and depression trials on (1) mean change from baseline on various ECG variables and on (2) incidence of patients meeting criteria for potentially important changes from baseline on various ECG variables revealed no important differences between fluvoxamine maleate and placebo.
6.8 Other Reactions Observed During the Premarketing Evaluation of Fluvoxamine
During premarketing clinical trials conducted in North America and Europe, multiple doses of fluvoxamine maleate extended-release capsules or immediate-release fluvoxamine maleate tablets were administered for a combined total of 3,219 patient exposures in patients suffering OCD or other studied disorders. These exposures include 482 patient exposures with fluvoxamine maleate extended-release capsules and 2,737 patient exposures with immediate-release fluvoxamine maleate tablets. Untoward reactions associated with this exposure were recorded by clinical investigators using descriptive terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse reactions without first grouping similar types of untoward reactions into a limited (i.e., reduced) number of standard reaction categories.
In the tabulations that follow, a COSTART-based Dictionary terminology has been used to classify reported adverse reactions. If the COSTART term for a reaction was so general as to be uninformative, it was replaced with a more informative term when possible. The frequencies presented, therefore, represent the proportion of the total patient exposures to multiple doses of fluvoxamine maleate who experienced a reaction of the type cited on at least one occasion while receiving fluvoxamine maleate. Reactions are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse reactions are defined as those occurring on one or more occasions in at least 1/100 patients; infrequent adverse reactions are those occurring between 1/100 and 1/1,000 patients; and rare adverse reactions are those occurring in less than 1/1,000 patients. It is important to emphasize that, although the events reported did occur during treatment with fluvoxamine maleate, a causal relationship to fluvoxamine maleate has not been established.
For fluvoxamine maleate extended-release capsules, all reported events are included in the list below, with the following exclusions: 1) those events already listed in Table 2 or previous sections of this prescribing information; 2) those events for which there is no basis to suspect a causal relationship; and 3) events that were reported in only one patient and judged not to be potentially serious.
Body as a Whole:Infrequent:chills, malaise, photosensitivity reaction, suicide attempt.
Cardiovascular System:Infrequent:syncope.
Digestive System:Infrequent:eructation, increased salivation.
Metabolic and Nutritional Disorders:Frequent:weight gain.
Nervous System: Infrequent:confusion, incoordination, sleep disorder, suicidal tendency.
Skin and Appendages:Infrequent:eczema, urticaria.
Special Senses:Infrequent:dry eyes, photophobia, taste loss.
Urogenital System: Infrequent:vaginal hemorrhage 1.
1Based on the number of females.
For immediate-release fluvoxamine tablets, all reported events are included in the list below, with the following exclusions: 1) those events already listed in Table 2, in previous sections of this prescribing information, or in the fluvoxamine maleate extended-release capsules list of Other Reactions Observed During Premarketing Evaluation; 2) those events for which there is no basis to suspect a causal relationship; and 3) events that were reported in only one patient and judged not to be potentially serious.
Body as a Whole:Infrequent:allergic reaction, neck pain, neck rigidity, overdose; Rare:sudden death.
Cardiovascular System:Frequent:hypotension; Infrequent:angina pectoris, bradycardia, cardiomyopathy, cardiovascular disease, cold extremities, conduction delay, myocardial infarction, pallor, pulse irregular, ST segment changes; Rare:AV block, cerebrovascular accident, embolus, pericarditis, phlebitis, pulmonary infarction, supraventricular extrasystoles.
Digestive System:Frequent:elevated liver transaminases; Infrequent:colitis, esophagitis, gastritis, gastroenteritis, gastrointestinal hemorrhage, gastrointestinal ulcer, glossitis, hemorrhoids, melena, rectal hemorrhage, stomatitis; Rare:biliary pain, cholecystitis, cholelithiasis, fecal incontinence, hematemesis, intestinal obstruction, jaundice.
Endocrine System:Infrequent:hypothyroidism; Rare:goiter.
Hemic and Lymphatic Systems:Infrequent:leukocytosis, lymphadenopathy, thrombocytopenia; Rare:leukopenia, purpura.
Metabolic and Nutritional Systems:Frequent:edema; Infrequent:dehydration, hypercholesterolemia; Rare:diabetes mellitus, hyperglycemia, hyperlipidemia, hypoglycemia, hypokalemia, lactate dehydrogenase increased.
Musculoskeletal System:Infrequent:arthralgia, arthritis, bursitis, generalized muscle spasm, myasthenia; Rare:myopathy.
Nervous System:Frequent:amnesia, apathy, hyperkinesia, hypokinesia, manic reaction, myoclonus, psychotic reaction; Infrequent:agoraphobia, akathisia, ataxia, CNS depression, convulsion, delirium, delusion, depersonalization, dyskinesia, dystonia, emotional lability, euphoria, extrapyramidal syndrome, gait unsteady, hallucinations, hemiplegia, hostility, hypersomnia, hypochondriasis, hypotonia, hysteria, increased libido, paralysis, paranoid reaction, phobia, psychosis, stupor, twitching, vertigo; Rare:akinesia, coma, fibrillations, mutism, obsessions, reflexes decreased, slurred speech, tardive dyskinesia, torticollis, trismus, withdrawal syndrome.
Respiratory System:Frequent:cough increased, sinusitis; Infrequent:asthma, bronchitis, hoarseness, hyperventilation; Rare:apnea, congestion of upper airway, hemoptysis, hiccups, laryngismus, obstructive pulmonary disease, pneumonia.
Skin:Infrequent:alopecia, dry skin, exfoliative dermatitis, furunculosis, seborrhea, skin discoloration.
Special Senses:Infrequent:accommodation abnormal, conjunctivitis, diplopia, eye pain, mydriasis, otitis media, parosmia, visual field defect; Rare:corneal ulcer.
Urogenital System:Infrequent:anuria, cystitis, delayed menstruation 1, dysuria, female lactation 1, hematuria, menopause 1, metrorrhagia 1, nocturia, premenstrual syndrome 1, urination impaired, vaginitis 1; Rare:kidney calculus, hematospermia 2, oliguria.
1Based on the number of females.
2Based on the number of males.
6.9 Postmarketing Reports
The following adverse reactions have been identified during post-approval use of immediate-release fluvoxamine maleate tablets or fluvoxamine maleate extended-release capsules. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. (Reactions that are discussed in other sections of this prescribing information are not repeated here.) These reactions include: activation syndrome, aggression, agranulocytosis, anaphylactic reaction, anger, blood glucose increased, bruxism, cardio-respiratory arrest, crying, dysarthria, dysphagia, electrocardiogram QT prolonged, fall, fatigue, feeling drunk, feeling jittery, gait disturbance, gastroesophageal reflux disease, glossodynia, hepatitis, homicidal ideation, impulsive behavior, ileus, inappropriate antidiuretic hormone secretion, interstitial lung disease, irritability, loss of consciousness, lethargy, muscular weakness, Parkinsonism, pancreatitis, pyrexia, renal impairment, rhabdomyolysis, self-injurious behavior, shock, somnolence neonatal, Stevens-Johnson syndrome, tachycardia, urinary retention, ventricular arrythmia, ventricular tachycardia (including torsades de pointes known to cause cardiac arrest, sometimes fatal), vision blurred, white blood cell count decreased, anosmia, and hyposmia.
-
7 DRUG INTERACTIONS
7.1 Potential Interactions with Drugs that Inhibit or are Metabolized by Cytochrome P450 Isoenzymes
Multiple hepatic cytochrome P450 isoenzymes are involved in the oxidative biotransformation of a large number of structurally different drugs and endogenous compounds. The available knowledge concerning the relationship of fluvoxamine and the cytochrome P450 isoenzyme system has been obtained mostly from pharmacokinetic interaction studies conducted in healthy volunteers, but some preliminary in vitrodata are also available. Based on a finding of substantial interactions of fluvoxamine with certain of these drugs [see later parts of this section and also Warnings and Precautions (5) for details ]and limited in vitrodata for CYP3A4, it appears that fluvoxamine inhibits several cytochrome P450 isoenzymes that are known to be involved in the metabolism of other drugs such as: CYP1A2 (e.g., warfarin, theophylline, propranolol, tizanidine), CYP2C9 (e.g., warfarin), CYP3A4 (e.g., alprazolam), and CYP2C19 (e.g., omeprazole).
In vitrodata suggest that fluvoxamine is a relatively weak inhibitor of CYP2D6.
Approximately 7% of the normal population has a genetic code that leads to reduced levels of activity of CYP2D6 enzyme. Such individuals have been referred to as “poor metabolizers” (PM) of drugs such as debrisoquin, dextromethorphan, and tricyclic antidepressants. While none of the drugs studied for drug interactions significantly affected the pharmacokinetics of fluvoxamine, an in vivostudy of fluvoxamine single-dose pharmacokinetics in 13 PM subjects demonstrated altered pharmacokinetic properties compared to 16 “extensive metabolizers” (EM): mean C max, AUC, and half-life were increased by 52%, 200%, and 62%, respectively, in the PM compared to the EM group. This suggests that fluvoxamine is metabolized, at least in part, by CYP2D6. Caution is indicated in patients known to have reduced levels of cytochrome P450 2D6 activity and those receiving concomitant drugs known to inhibit this cytochrome P450 isoenzyme (e.g., quinidine).
The metabolism of fluvoxamine has not been fully characterized and the effects of potent cytochrome P450 isoenzyme inhibition, such as the ketoconazole inhibition of CYP3A4, on fluvoxamine metabolism have not been studied.
A clinically significant fluvoxamine interaction is possible with drugs having a narrow therapeutic ratio such as pimozide, warfarin, theophylline, certain benzodiazepines, omeprazole, and phenytoin. If fluvoxamine maleate extended-release capsules are to be administered together with a drug that is eliminated via oxidative metabolism and has a narrow therapeutic window, plasma levels and/or pharmacodynamic effects of the latter drug should be monitored closely, at least until steady-state conditions are reached [see Contraindications (4) and Warnings and Precautions (5)] .
7.2 CNS Active Drugs
Antipsychotics:See Warningsand Precautions( 5.2) .
Benzodiazepines:See Warningsand Precautions ( 5.9) .
Alprazolam:See Warningsand Precautions ( 5.9) .
Diazepam:See Warnings and Precautions ( 5.9) .
Lorazepam:A study of multiple doses of immediate-release fluvoxamine maleate tablets (50 mg given twice daily) in healthy male volunteers (N = 12) and a single dose of lorazepam (4 mg single dose) indicated no significant pharmacokinetic interaction. On average, both lorazepam alone and lorazepam with fluvoxamine produced substantial decrements in cognitive functioning; however, the co-administration of fluvoxamine and lorazepam did not produce larger mean decrements compared to lorazepam alone.
Alcohol:Studies involving single 40 g doses of ethanol (oral administration in one study and intravenous in the other) and multiple dosing with immediate-release fluvoxamine maleate tablets (50 mg given twice daily) revealed no effect of either drug on the pharmacokinetics or pharmacodynamics of the other. As with other psychotropic medications, patients should be advised to avoid alcohol while taking fluvoxamine maleate extended-release capsules.
Carbamazepine:Elevated carbamazepine levels and symptoms of toxicity have been reported with the co-administration of immediate-release fluvoxamine maleate tablets and carbamazepine.
Clozapine:See Warnings and Precautions ( 5.9) .
Lithium:As with other serotonergic drugs, lithium may enhance the serotonergic effects of fluvoxamine and, therefore, the combination should be used with caution. Seizures have been reported with the co-administration of immediate-release fluvoxamine maleate tablets and lithium.
Methadone:See Warnings and Precautions ( 5.9) .
Monoamine OxidaseInhibitors:See Contraindications ( 4.1) and Warnings and Precautions ( 5.2) .
Pimozide:See Contraindications ( 4) and Warnings and Precautions ( 5.6) .
Ramelteon:See Contraindications( 4) and Warnings and Precautions ( 5.8) .
Serotonergic Drugs:See Warnings and Precautions ( 5.2).
Tacrine:In a study of 13 healthy, male volunteers, a single 40 mg dose of tacrine added to immediate-release fluvoxamine maleate tablets 100 mg/day administered at steady-state was associated with 5- and 8-fold increases in tacrine C maxand AUC, respectively, compared to the administration of tacrine alone. Five subjects experienced nausea, vomiting, sweating, and diarrhea following co-administration, consistent with the cholinergic effects of tacrine.
Thioridazine:See Contraindications( 4) and Warnings and Precautions ( 5.4) .
Tizanidine:See Contraindications ( 4) and Warnings and Precautions ( 5.5) .
Tricyclic Antidepressants (TCAs):Significantly increased plasma TCA levels have been reported with the co-administration of immediate-release fluvoxamine maleate tablets and amitriptyline, clomipramine or imipramine. Caution is indicated with the co-administration of fluvoxamine maleate extended-release capsules and TCAs; plasma TCA concentrations may need to be monitored, and the dose of TCA may need to be reduced.
Triptans:There have been rare postmarketing reports of serotonin syndrome with use of an SSRI and a triptan. If concomitant treatment of fluvoxamine maleate extended-release capsules with a triptan is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases [see Warnings and Precautions ( 5.2)] .
Sumatriptan-There have been rare postmarketing reports describing patients with weakness, hyperreflexia, and incoordination following the use of a selective serotonin reuptake inhibitor (SSRI) and sumatriptan. If concomitant treatment with sumatriptan and an SSRI (e.g., fluoxetine, fluvoxamine, paroxetine, sertraline) is clinically warranted, appropriate observation of the patient is advised.
Tryptophan:Tryptophan may enhance the serotonergic effects of fluvoxamine, and the combination should, therefore, be used with caution. Severe vomiting has been reported with the co-administration of immediate-release fluvoxamine maleate tablets and tryptophan [see Warnings and Precautions ( 5.2)] .
7.3 Other Drugs
Alosetron: See Contraindications (4), Warnings and Precautions (5.7),and Lotronex ®(alosetron) package insert.
Digoxin:Administration of immediate-release fluvoxamine maleate tablets 100 mg daily for 18 days (N = 8) did not significantly affect the pharmacokinetics of a 1.25 mg single intravenous dose of digoxin.
Diltiazem:Bradycardia has been reported with the co-administration of immediate-release fluvoxamine maleate tablets and diltiazem.
Mexiletine: See Warnings and Precautions (5.9).
Propranolol and Other Beta-Blockers:Co-administration of immediate-release fluvoxamine maleate tablets 100 mg per day and propranolol 160 mg per day in normal volunteers resulted in a mean five-fold increase (range 2 to 17) in minimum propranolol plasma concentrations. In this study, there was a slight potentiation of the propranolol-induced reduction in heart rate and reduction in the exercise diastolic pressure.
One case of bradycardia and hypotension and a second case of orthostatic hypotension have been reported with the co-administration of immediate-release fluvoxamine maleate tablets and metoprolol.
If propranolol or metoprolol is co-administered with fluvoxamine maleate extended-release capsules, a reduction in the initial beta-blocker dose and more cautious dose titration are recommended. No dosage adjustment is required for fluvoxamine maleate extended-release capsules.
Co-administration of immediate-release fluvoxamine maleate tablets 100 mg per day with atenolol 100 mg per day (N = 6) did not affect the plasma concentrations of atenolol. Unlike propranolol and metoprolol, which undergo hepatic metabolism, atenolol is eliminated primarily by renal excretion.
Theophylline: See Warnings and Precautions (5.9).
Warfarin and Other Drugs that Interfere with Hemostasis (NSAIDs, Aspirin, etc.): See Warnings and Precautions (5.9, 5.11).
7.4 Effects of Smoking on Fluvoxamine Metabolism
Smokers had a 25% increase in the metabolism of fluvoxamine compared to non-smokers.
7.5 Electroconvulsive Therapy (ECT)
There are no clinical studies establishing the benefits or risks of combined use of ECT and fluvoxamine maleate.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects – Pregnancy Category C:When pregnant rats were given daily doses of fluvoxamine (60, 120, or 240 mg/kg) orally throughout the period of organogenesis, developmental toxicity in the form of increased embryofetal death and increased incidences of fetal eye abnormalities (folded retinas) was observed at doses of 120 mg/kg or greater. Decreased fetal body weight was seen at the high dose. The no effect dose for developmental toxicity in this study was 60 mg/kg (approximately 2 times the maximum recommended human dose [MRHD] on a mg/m 2basis).
In a study in which pregnant rabbits were administered doses of up to 40 mg/kg (approximately 2 times the MRHD on a mg/m 2basis) orally during organogenesis, no adverse effects on embryofetal development were observed.
In other reproduction studies in which female rats were dosed orally during pregnancy and lactation (5, 20, 80, or 160 mg/kg), increased pup mortality at birth was seen at doses of 80 mg/kg or greater and decreases in pup body weight and survival were observed at all doses (low effect dose approximately 0.1 times the MRHD on a mg/m 2basis).
Nonteratogenic Effects: Based on data from published observational studies, exposure to SSRIs, particularly in the month before delivery, has been associated with a less than 2-fold increase in the risk of postpartum hemorrhage [see Warnings and Precautions ( 5.11) and Clinical Considerations] .
Neonates exposed to fluvoxamine maleate tablets and other SSRIs or serotonin and norepinephrine reuptake inhibitors (SNRIs) late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability and constant crying. These features are consistent with either a direct toxic effect of SSRIs or SNRIs or, possibly, a drug discontinuation syndrome. It should be noted that, in some cases, the clinical picture is consistent with serotonin syndrome [see Warnings and Precautions-Serotonin Syndrome ( 5.2)] .
Infants exposed to SSRIs in pregnancy may have an increased risk for persistent pulmonary hypertension of the newborn (PPHN). PPHN occurs in 1 to 2 per 1,000 live births in the general population and is associated with substantial neonatal morbidity and mortality. Several recent epidemiologic studies suggest a positive statistical association between SSRI use (fluvoxamine maleate immediate-release tablets and fluvoxamine maleate extended-release capsules are SSRIs) in pregnancy and PPHN. Other studies do not show a significant statistical association.
Physicians should also note the results of a prospective longitudinal study of 201 pregnant women with a history of major depression, who were either on antidepressants or had received antidepressants less than 12 weeks prior to their last menstrual period, and were in remission. Women who discontinued antidepressant medication during pregnancy showed a significant increase in relapse of their major depression compared to those women who remained on antidepressant medication throughout pregnancy.
When treating a pregnant woman with fluvoxamine, the physician should carefully consider both the potential risks of taking an SSRI, along with the established benefits of treating depression with an antidepressant. This decision can only be made on a case-by-case basis (see DOSAGE AND ADMINISTRATION (2.7)] .
Maternal Adverse Reactions:Use of fluvoxamine maleate extended-release capsules in the month before delivery may be associated with an increased risk of postpartum hemorrhage [see Warnings and Precautions ( 5.11)].
8.3 Nursing Mothers
Fluvoxamine is secreted in human breast milk. Because of the potential for serious adverse reactions in nursing infants from fluvoxamine maleate extended-release capsules, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Fluvoxamine maleate extended-release capsules have not been evaluated in pediatric patients (see Boxed Warning). The efficacy of fluvoxamine maleate administered as immediate-release tablets for the treatment of OCD was demonstrated in a 10-week multi-center placebo-controlled study with 120 outpatients ages 8 to 17. In addition, 99 of these outpatients continued open-label fluvoxamine maleate treatment for up to another one to three years, equivalent to 94 patient years. The adverse reaction profile observed in that study was generally similar to that observed in adult studies with immediate-release fluvoxamine maleate tablets [see Adverse Reactions (6.3)].
Decreased appetite and weight loss have been observed in association with the use of fluvoxamine as well as other SSRIs. Consequently, regular monitoring of weight and growth should be performed in children and adolescents treated with an SSRI such as fluvoxamine maleate extended-release capsules.
The risks, if any, that may be associated with fluvoxamine’s extended use in children and adolescents with OCD have not been systematically assessed. The prescriber should be mindful that the evidence relied upon to conclude that fluvoxamine is safe for use in children and adolescents derives from relatively short-term clinical studies and from extrapolation of experience gained with adult patients. In particular, there are no studies that directly evaluate the effects of long-term fluvoxamine use on the growth, cognitive behavioral development, and maturation of children and adolescents. Although there is no affirmative finding to suggest that fluvoxamine possesses a capacity to adversely affect growth, development, or maturation, the absence of such findings is not compelling evidence of the absence of the potential of fluvoxamine to have adverse effects in chronic use [see Warnings and Precautions- Clinical Worsening and Suicide Risk (5.1)] .
Safety and effectiveness in the pediatric population other than pediatric patients with OCD have not been established [see Boxed Warning and Warnings and Precautions- Clinical Worsening and Suicide Risk (5.1)]. Anyone considering the use of fluvoxamine maleate extended-release capsules in a child or adolescent must balance the potential risks with the clinical need.
8.5 Geriatric Use
Approximately 230 patients and 5 patients participating in controlled premarketing studies with immediate-release fluvoxamine maleate tablets and fluvoxamine maleate extended-release capsules, respectively, were 65-years of age or over. No overall differences in safety were observed between these patients and younger patients. Other reported clinical experience has not identified differences in response between the elderly and younger patients. However, SSRIs and SNRIs, including fluvoxamine, have been associated with several cases of clinically significant hyponatremia in elderly patients, who may be at greater risk for this adverse reaction [see Warnings and Precautions ( 5.14)] . Furthermore, the clearance of fluvoxamine is decreased by about 50% in elderly compared to younger patients [see Clinical Pharmacology–Elderly ( 12.3)] , and greater sensitivity of some older individuals also cannot be ruled out. Consequently, a lower starting dose should be considered in elderly patients, and fluvoxamine maleate extended-release capsules should be slowly titrated during initiation of therapy.
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance Class
Fluvoxamine maleate extended-release capsules are not a controlled substance.
9.2 Physical and Psychological Dependence
The potential for abuse, tolerance, and physical dependence with immediate-release fluvoxamine maleate has been studied in a non-human primate model. No evidence of dependency phenomena was found. The discontinuation effects of fluvoxamine maleate extended-release capsules were not systematically evaluated in controlled clinical trials. Fluvoxamine maleate extended-release capsules were not systematically studied in clinical trials for potential for abuse, but there was no indication of drug-seeking behavior in clinical trials. It should be noted, however, that patients at risk for drug dependency were systematically excluded from investigational studies of immediate-release fluvoxamine maleate. Generally, it is not possible to predict on the basis of preclinical or premarketing clinical experience the extent to which a CNS active drug will be misused, diverted, and/or abused once marketed. Consequently, health care providers should carefully evaluate patients for a history of drug abuse and follow such patients closely, observing them for signs of fluvoxamine maleate extended-release capsules misuse or abuse (i.e., development of tolerance, incrementation of dose, drug-seeking behavior).
-
10 OVERDOSAGE
10.1 Human Experience
Exposure to immediate-release fluvoxamine maleate tablets includes over 45,000 patients treated in clinical trials and an estimated exposure of 50,000,000 patients treated during worldwide marketing experience (end of 2005). Of the 539 cases of deliberate or accidental overdose involving fluvoxamine reported from this population, there were 55 deaths. Of these, 9 were in patients thought to be taking immediate-release fluvoxamine tablets alone and the remaining 46 were in patients taking fluvoxamine along with other drugs. Among non-fatal overdose cases, 404 patients recovered completely. Five patients experienced adverse sequelae of overdosage, to include persistent mydriasis, unsteady gait, hypoxic encephalopathy, kidney complications (from trauma associated with overdose), bowel infarction requiring a hemicolectomy, and vegetative state. In 13 patients, the outcome was provided as abating at the time of reporting. In the remaining 62 patients, the outcome was unknown. The largest known ingestion of fluvoxamine immediate‑release tablets involved 12,000 mg (equivalent to 2 to 3 months’ dosage). The patient fully recovered. However, ingestions as low as 1,400 mg have been associated with lethal outcome, indicating considerable prognostic variability.
In the controlled clinical trials with 403 patients treated with fluvoxamine maleate extended‑release capsules, there was one nonfatal intentional overdose.
Commonly (≥ 5%) observed adverse reactions associated with fluvoxamine maleate overdose include gastrointestinal complaints (nausea, vomiting, and diarrhea), coma, hypokalemia, hypotension, respiratory difficulties, somnolence, and tachycardia. Other notable signs and symptoms seen with immediate-release fluvoxamine maleate overdose (single or multiple drugs) include bradycardia, ECG abnormalities (such as heart arrest, QT interval prolongation, first degree atrioventricular block, bundle branch block, and junctional rhythm), convulsions, dizziness, liver function disturbances, tremor, and increased reflexes.
10.2 Management of Overdosage
Treatment should consist of those general measures employed in the management of overdosage with any antidepressant.
Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. General supportive and symptomatic measures are also recommended. Induction of emesis is not recommended. Gastric lavage with a large-bore orogastric tube with appropriate airway protection, if needed, may be indicated if performed soon after ingestion, or in symptomatic patients.
Activated charcoal should be administered. Due to the large volume of distribution of this drug, forced diuresis, dialysis, hemoperfusion, and exchange transfusion are unlikely to be of benefit. No specific antidotes for fluvoxamine are known.
A specific caution involves patients taking, or recently having taken, fluvoxamine maleate who might ingest excessive quantities of a tricyclic antidepressant. In such a case, accumulation of the parent tricyclic and/or an active metabolite may increase the possibility of clinically significant sequelae and extend the time needed for close medical observation [see Drug Interactions ( 7.2).
In managing overdosage, consider the possibility of multiple drug involvement. The health care provider should consider contacting a poison control center for additional information on the treatment of any overdose. Telephone numbers for certified poison control centers are listed in the Physicians’ Desk Reference(PDR).
-
11 DESCRIPTION
Fluvoxamine maleate extended-release capsules for oral administration contain fluvoxamine maleate, USP, a selective serotonin (5-HT) reuptake inhibitor (SSRI) belonging to the chemical series, the 2-aminoethyl oxime ethers of aralkylketones.
Fluvoxamine maleate, USP is chemically designated as 5-methoxy-4'-(trifluoromethyl) valerophenone-(E)-O-(2-aminoethyl)oxime maleate (1:1) and has the molecular formula C 15H 21O 2N 2F 3•C 4H 4O 4. Its molecular weight is 434.4.
The structural formula is:
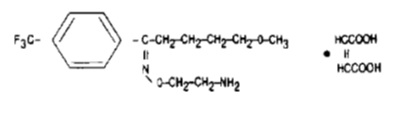
Fluvoxamine maleate, USP is a white to off-white crystalline powder that is sparingly soluble in water, freely soluble in ethanol (96%) and chloroform, and practically insoluble in diethyl ether.
Fluvoxamine maleate extended-release capsules are available in 100 mg and 150 mg strengths for oral administration. In addition to the active ingredient, fluvoxamine maleate, USP each capsule contains the following inactive ingredients: D&C Red 28, ethylcellulose, FD&C Blue 1, gelatin, hydroxypropyl cellulose, hypromellose, povidone, sugar spheres, triethyl citrate, and titanium dioxide. The capsules are printed with edible ink containing black iron oxide, potassium hydroxide, propylene glycol and shellac.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of fluvoxamine maleate in obsessive compulsive disorder is presumed to be linked to its specific serotonin reuptake inhibition in brain neurons. Fluvoxamine has been shown to be a potent inhibitor of the serotonin reuptake transporter in preclinical studies, both in vitroand in vivo.
12.2 Pharmacodynamics
In in vitrostudies, fluvoxamine maleate had no significant affinity for histaminergic, alpha or beta adrenergic, muscarinic, or dopaminergic receptors. Antagonism of some of these receptors is thought to be associated with various sedative, cardiovascular, anticholinergic, and extrapyramidal effects of some psychotropic drugs.
12.3 Pharmacokinetics
Bioavailability:A single-dose crossover study in 28 healthy subjects was conducted to compare the pharmacokinetics of fluvoxamine after administration of fluvoxamine maleate extended-release capsules and immediate-release fluvoxamine maleate tablets.
In the single-dose crossover study, mean C maxwas 38% lower and relative bioavailability was 84% for fluvoxamine maleate extended-release capsules versus immediate-release fluvoxamine maleate tablets.
In a multiple-dose proportionality study, fluvoxamine maleate extended-release capsules were administered over a dose range of 100 mg/day to 300 mg/day to 20 healthy volunteers. Steady-state plasma concentrations were achieved within a week of dosing. Mean maximum plasma concentrations were 47 ng/mL, 161 ng/mL, and 319 ng/mL, respectively, at the 100 mg, 200 mg, and 300 mg administered dose levels. Fluvoxamine exhibited non-linear pharmacokinetics producing disproportionately higher concentrations over the dose range. The AUC and C maxvalues increased 5.7-fold following the 3-fold increase in dose from 100 mg to 300 mg.
Food caused the mean AUC and C maxof fluvoxamine to increase only slightly; therefore, administration of fluvoxamine maleate extended-release capsules with food does not significantly affect the absorption of fluvoxamine.
Distribution/Protein Binding:The mean apparent volume of distribution for fluvoxamine is approximately 25 L/kg, suggesting extensive tissue distribution.
Approximately 80% of fluvoxamine is bound to plasma protein, mostly albumin, over a concentration range of 20 to 2,000 ng/mL.
Metabolism:Fluvoxamine maleate is extensively metabolized by the liver; the main metabolic routes are oxidative demethylation and deamination. Nine metabolites were identified following a 5 mg radiolabelled dose of fluvoxamine maleate, constituting approximately 85% of the urinary excretion products of fluvoxamine. The main human metabolite was fluvoxamine acid which, together with its N-acetylated analog, accounted for about 60% of the urinary excretion products. A third metabolite, fluvoxethanol, formed by oxidative deamination, accounted for about 10%. Fluvoxamine acid and fluvoxethanol were tested in an in vitroassay of serotonin and norepinephrine reuptake inhibition in rats; they were inactive except for a weak effect of the former metabolite on inhibition of serotonin uptake (1 to 2 orders of magnitude less potent than the parent compound). Approximately 2% of fluvoxamine was excreted in urine unchanged [see Drug Interactions (7)] .
Elimination:Following a 14C-labelled oral dose of fluvoxamine maleate (5 mg), an average of 94% of drug-related products was recovered in the urine within 71 hours.
After administration of a 100 mg, single oral dose of fluvoxamine maleate extended-release capsules, the mean plasma half-life of fluvoxamine in healthy male and female volunteers was 16.3 hours.
Gender:In a study with 15 male and 13 female healthy volunteers who were administered fluvoxamine maleate extended-release capsules 100 mg, AUC and C maxof fluvoxamine were increased by approximately 60% in females compared to males. There were no differences in the elimination half-life between males and females.
Elderly Subjects:In a study using immediate-release fluvoxamine maleate tablets at 50 mg and 100 mg and comparing elderly (ages 66 to 73 years) and young subjects (ages 19 to 35 years), mean maximum plasma concentrations in the elderly were 40% higher. The multiple-dose elimination half-life of fluvoxamine was 17.4 hours and 25.9 hours in the elderly compared to 13.6 hours and 15.6 hours in the young subjects at steady state for 50 mg and 100 mg doses, respectively.
In elderly patients administered immediate-release fluvoxamine maleate tablets, the clearance of fluvoxamine was reduced by about 50%; therefore, fluvoxamine maleate extended-release capsules should be slowly titrated during initiation of therapy [see Dosage and Administration (2.3)] .
Pediatric Subjects:The pharmacokinetics of fluvoxamine maleate extended-release capsules have not been evaluated in pediatric patients. However, the multiple-dose pharmacokinetics of fluvoxamine were determined in male and female children (ages 6 to 11) and adolescents (ages 12 to 17). Steady-state plasma fluvoxamine concentrations were 2- to 3-fold higher in children than in adolescents. AUC and C maxin children were 1.5- to 2.7-fold higher than that in adolescents (see Table 4). As in adults, both children and adolescents exhibited non-linear multiple-dose pharmacokinetics. Female children showed significantly higher AUC (0-12)and C maxcompared to male children and, therefore, lower doses of immediate-release fluvoxamine maleate tablets may produce therapeutic benefit (see Table 5). No gender differences were observed in adolescents. Steady-state plasma fluvoxamine concentrations were similar in adults and adolescents at a dose of 300 mg/day, indicating that fluvoxamine exposure was similar in these two populations (see Table 4). Dose adjustment in adolescents (up to the adult maximum dose of 300 mg) may be indicated to achieve therapeutic benefit.
TABLE 4 COMPARISON OF MEAN (SD) IMMEDIATE-RELEASE TABLET FLUVOXAMINE MALEATE PHARMACOKINETIC PARAMETERS BETWEEN CHILDREN, ADOLESCENTS, AND ADULTS Pharmacokinetic Parameter Dose = 200 mg/day Dose = 300 mg/day (body weight corrected) (100 mg Twice Daily) (150 mg Twice Daily) Children Adolescent Adolescent Adult (n = 10) (n = 17) (n= 13) (n = 16) AUC 0-12(ng •h/mL/kg) 155.1 (160.9) 43.9 (27.9) 69.6 (46.6) 59.4 (40.9) C max(ng/mL/kg) 14.8 (14.9) 4.2 (2.6) 6.7 (4.2) 5.7 (3.9) C min(ng/mL/kg) 11.0 (11.9) 2.9 (2.0) 4.8 (3.8) 4.6 (3.2) TABLE 5 COMPARISON OF MEAN (SD) IMMEDIATE-RELEASE TABLET FLUVOXAMINE MALEATE PHARMACOKINETIC PARAMETERS BETWEEN MALE AND FEMALE CHILDREN (6 TO 11 YEARS) Dose = 200 mg/day (100 mg Twice Daily) Pharmacokinetic Parameter Male Children Female Children (body weight corrected) (n = 7) (n = 3) AUC 0-12(ng •h/mL/kg) 95.8 (83.9) 293.5 (233.0) C max(ng/mL/kg) 9.1 (7.6) 28.1 (21.1) C min(ng/mL/kg) 6.6 (6.1) 21.2 (17.6) Hepatic and Renal Disease:A cross-study comparison (healthy subjects versus patients with hepatic dysfunction) using immediate-release fluvoxamine maleate tablets suggested a 30% decrease in fluvoxamine clearance in association with hepatic dysfunction. The mean minimum plasma concentrations in renally impaired patients (creatinine clearance of 5 mL/min to 45 mL/min) after 4 weeks and 6 weeks of treatment (50 mg given twice daily, N = 13) were comparable to each other, suggesting no accumulation of fluvoxamine in these patients [see Warnings and Precautions–Use in Patients with Concomitant Illness (5.15)] .
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis:There was no evidence of carcinogenicity in rats treated orally with fluvoxamine maleate for 30 months or hamsters treated orally with fluvoxamine maleate for 20 months (females) or 26 months (males). The daily doses in the high-dose groups in these studies were increased over the course of the study from a minimum of 160 mg/kg to a maximum of 240 mg/kg in rats, and from a minimum of 135 mg/kg to a maximum of 240 mg/kg in hamsters. The maximum dose of 240 mg/kg is approximately 6 times the maximum recommended human dose (MRHD) on a mg/m 2basis.
Mutagenesis:No evidence of genotoxic potential was observed in a mouse micronucleus test, an in vitrochromosome aberration test, or the Ames microbial mutagen test with or without metabolic activation.
Impairment of Fertility:In a study in which male and female rats were administered fluvoxamine (60, 120, or 240 mg/kg) orally prior to and during mating and gestation, fertility was impaired at doses of 120 mg/kg or greater, as evidenced by increased latency to mating, decreased sperm count, decreased epididymal weight, and decreased pregnancy rate. In addition, the numbers of implantations and embryos were decreased at the highest dose. The no effect dose for fertility impairment was 60 mg/kg (approximately 2 times the MRHD on a mg/m 2basis).
-
14 CLINICAL STUDIES
14.1 Obsessive Compulsive Disorder (OCD)
The effectiveness of fluvoxamine maleate extended-release capsules for the treatment of OCD was demonstrated in a 12-week, multi-center, placebo-controlled study of adult outpatients. Patients in this trial were titrated in 50 mg increments over the first six weeks of the study on the basis of response and tolerance from a dose of 100 mg/day to a fluvoxamine maleate dose within a range of 100 mg to 300 mg once-a-day. Patients in this study had moderate to severe OCD (DSM-IV), with mean baseline ratings on the Yale-Brown Obsessive Compulsive Scale (Y-BOCS), total scores of 26.6 and 26.3 for fluvoxamine and placebo-treatment groups, respectively.
Patients receiving fluvoxamine maleate extended-release capsules demonstrated statistically significant improvement over placebo patients at the primary endpoint (Week 12) compared to baseline on the Y-BOCS. The mean daily dose of fluvoxamine maleate extended-release capsules administered to patients was 261 mg at end of study.
Exploratory analyses for age and gender effects on outcomes did not show any significant differential responsiveness on the basis of age or sex.
The effectiveness of immediate-release fluvoxamine maleate tablets for the treatment of OCD was demonstrated in two 10-week multi-center, parallel group studies of adult outpatients. Patients in these trials were titrated to a total daily fluvoxamine maleate dose of 150 mg/day over the first two weeks of the trial, after which the dose was adjusted within a range of 100 to 300 mg/day (given in two doses per day), on the basis of response and tolerance. Patients in these studies had moderate to severe OCD (DSM-III-R), with mean baseline ratings on the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) total score of 23.
14.2 Adult OCD Maintenance Study with Immediate-Release Fluvoxamine Maleate Tablets
In a maintenance trial of adult outpatients with OCD, 114 patients meeting DSM-IV criteria for OCD and with a Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score ≥ 18 were titrated to an effective dose of immediate-release fluvoxamine maleate tablets 100 to 300 mg/day as part of an initial 10-week single-blind treatment phase. Treatment response during this single-blind phase was defined as Y-BOCS scores at least 30% lower than baseline at the end of weeks 8 and 10. Of the patients who responded, their average duration of response was 4 weeks. Patients who responded during this initial phase were randomized either to continuation of immediate-release fluvoxamine maleate tablets (N = 56) or to placebo (N = 58) in a double-blind phase for observation of relapse. Relapse during the double-blind phase was defined as an increase in the Y-BOCS score of at least 30% over the baseline for that phase or patient refusal to continue treatment due to a substantial increase in OCD symptoms. In the double-blind phase, patients receiving continued immediate-release fluvoxamine maleate tablets treatment experienced, on average, a significantly lower relapse rate than those receiving placebo.
An examination of population subgroups from this trial did not reveal any clear evidence of a differential maintenance effect on the basis of age or gender.
14.3 Pediatric OCD Study
Fluvoxamine maleate extended-release capsules have not been evaluated in pediatric patients. However, the effectiveness of immediate-release fluvoxamine maleate tablets for the treatment of OCD was demonstrated in a 10-week multi-center, parallel group study in a pediatric outpatient population (children and adolescents, ages 8 to 17 years). Patients in this study were titrated to a total daily fluvoxamine dose of approximately 100 mg/day over the first two weeks of the trial, following which the dose was adjusted within a range of 50 to 200 mg/day (given in two doses per day) on the basis of response and tolerance. All patients had moderate-to-severe OCD (DSM-III-R) with mean baseline ratings on the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) total score of 24.
Post hoc exploratory analyses for gender effects on outcomes did not suggest any differential responsiveness on the basis of gender. Further exploratory analyses revealed a prominent treatment effect in the 8 year to 11 year age group and essentially no effect in the 12 year to 17 year age group. While the significance of these results is not clear, the 2 to 3 fold higher steady-state plasma fluvoxamine concentrations in children compared to adolescents [see Clinical Pharmacology–Pediatric Subjects (12.3)] is suggestive that decreased exposure in adolescents may have been a factor, and dose adjustment in adolescents (up to the adult maximum dose of 300 mg/day) may be indicated to achieve therapeutic benefit.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Fluvoxamine maleate extended-release capsules are available in the following strengths, colors, imprints, and presentations:
100 mg Extended-Release Capsules:Available in a size “2” hard gelatin capsule with purple opaque cap imprinted with “FL” in black ink and white opaque body imprinted with “100” in black ink, filled with white to off-white spherical pellets.
Bottles of 30 with child-resistant closure………NDC 69452-182-13
Bottles of 1000…………………………………NDC 69452-182-32
150 mg Extended-Release Capsules:Available in a size “1” hard gelatin capsule with purple opaque cap imprinted with “FL” in black ink and white opaque body imprinted with “150” in black ink, filled with white to off-white spherical pellets.
Bottles of 30 with child-resistant closure ………NDC 69452-183-13
Bottles of 1000………………………………… NDC 69452-183-32
16.2 Storage
Keep out of reach of children.
Fluvoxamine maleate extended-release capsules should be stored at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Protect from high humidity.
Avoid exposure to temperatures above 30°C (86°F).
Dispense in tight containers.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling ( Medication Guide).
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with fluvoxamine maleate extended-release capsules and should counsel them in the appropriate use. A patient Medication Guide discussing antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions and other important information about fluvoxamine maleate extended-release capsules is available for fluvoxamine maleate extended-release capsules. The prescriber or health professional should instruct patients, their families, and their caregivers to read both sections of the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking fluvoxamine maleate extended-release capsules.
17.1 Clinical Worsening and Suicide Risk
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient’s prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient’s presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate the need for very close monitoring and possibly changes in the medication [see Boxed Warnin g and Warnings and Precautions (5.1)].
17.2 Serotonin Syndrome
Patients should be cautioned about the risk of serotonin syndrome particularly with the concomitant use of fluvoxamine with other serotonergic agents (including triptans, tricyclic antidepressants, opioids, lithium, tryptophan, buspirone, amphetamines, and St. John’s Wort) [see Warnings and Precautions ( 5.2) ].
17.3 Contraindicated Medications
Patients should be advised that the following medications should not be used while taking fluvoxamine maleate extended-release capsules:
- Monoamine oxidase inhibitors (MAOIs): See Contraindications ( 4.1) and Warnings and Precautions ( 5.2) .
- Thioridazine:See Contraindications( 4) and Warnings and Precautions ( 5.4) .
- Tizanidine:See Contraindications( 4) and Warnings and Precautions ( 5.5) .
- Pimozide:See Contraindications( 4) and Warnings and Precautions ( 5.6) .
- Alosetron:See Contraindications( 4) and Warnings and Precautions ( 5.7) .
- Ramelteon:See Contraindications( 4) and Warnings and Precautions ( 5.8) .
In addition, MAOIs should not be taken within 14 days (2 weeks) after stopping fluvoxamine maleate extended-release capsules, and fluvoxamine maleate extended-release capsules should not be taken within two weeks after stopping treatment with an MAOI [see Contraindications (4.1)and Warnings and Precautions (5.2)].
17.4 Other Potentially Hazardous Drug Interactions
Patients should be advised that the use of fluvoxamine maleate extended-release capsules with any of the following medications may produce clinically significant adverse reactions. Patients should inform their physician if they are taking any of these medications before starting treatment with fluvoxamine maleate extended-release capsules. Patients should also inform their physician prior to taking any of these medications while receiving fluvoxamine maleate extended-release capsule therapy.
- Serotonergic drugs, including triptans, tramadol, and tryptophan:See Warnings and Precautions ( 5.2) .
- Antipsychotic agents, including clozapine:See Warnings and Precautions ( 5.2, 5.9) .
- Certain benzodiazepines:See Warnings and Precautions ( 5.9) .
- Methadone:See Warnings and Precautions ( 5.9) .
- Mexiletine: See Warnings and Precautions ( 5.9) .
- Theophylline:See Warnings and Precautions ( 5.9) .
- Warfarin and other drugs that interfere with hemostasis:Patients should be cautioned about the concomitant use of fluvoxamine and NSAIDs, aspirin, or other drugs that affect coagulation since the combined use of psychotropic drugs that interfere with serotonin reuptake and these agents has been associated with an increased risk of bleeding [see Warnings and Precautions (5.9, 5.11)].
- Diuretics:See Warnings and Precautions ( 5.14) .
In addition, patients should be advised to notify their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs, since there is a potential for clinically important interactions with fluvoxamine maleate extended-release capsules.
17.5 Abnormal Bleeding
Patients should be advised that fluvoxamine maleate extended-release capsules may increase the risk of bleeding events, which have ranged from ecchymoses, hematomas, epistaxis, and petechiae to life-threatening hemorrhages. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs, warfarin, and other anticoagulants may add to this risk [see Warnings and Precautions (5.9, 5.11)].
17.6 Angle Closure Glaucoma
Patients should be advised that taking fluvoxamine maleate extended-release capsules can cause mild pupillary dilation, which in susceptible individuals, can lead to an episode of angle closure glaucoma. Pre-existing glaucoma is almost always open-angle glaucoma because angle closure glaucoma, when diagnosed, can be treated definitively with iridectomy. Open-angle glaucoma is not a risk factor for angle closure glaucoma. Patients may wish to be examined to determine whether they are susceptible to angle closure, and have a prophylactic procedure (e.g., iridectomy), if they are susceptible [see Warnings and Precautions (5.3)] .
17.7 Interference with Cognitive or Motor Performance
Since any psychoactive drug may impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are certain that fluvoxamine maleate extended-release capsules therapy does not adversely affect their ability to engage in such activities.
17.8 Pregnancy
Patients should be advised to notify their physicians if they become pregnant or intend to become pregnant during therapy with fluvoxamine maleate extended-release capsules [see Use in Specific Populations (8.1)] .
17.9 Nursing
Patients receiving fluvoxamine maleate extended-release capsules should be advised to notify their physicians if they are breast-feeding an infant. [see Use in Specific Populations Nursing Mothers (8.3)] .
17.10 Alcohol
As with other psychotropic medications, patients should be advised to avoid alcohol while taking fluvoxamine maleate extended-release capsules.
17.11 Allergic Reactions
Patients should be advised to notify their physicians if they develop a rash, hives, or a related allergic phenomenon during therapy with fluvoxamine maleate extended-release capsules.
17.12 Sexual Dysfunction
Advise patients that use of fluvoxamine maleate extended-release capsules may cause symptoms of sexual dysfunction in both male and female patients. Inform patients that they should discuss any changes in sexual function and potential management strategies with their healthcare provider [see Warnings and Precautions ( 5.17)] .
Brands listed are the trademarks of their respective owners.
Distributed by:
Bionpharma Inc.
Princeton, NJ 08540
MADE IN INDIA
October 2023FDA-05
948026814
-
MEDICATION GUIDE
Fluvoxamine Maleate(floo vox' a meen mal' ee ate)
Extended-Release Capsules
Read the Medication Guide that comes with fluvoxamine maleate extended-release capsules before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or treatment. Talk to your healthcare provider if there is something you do not understand or want to learn more about.
What is the most important information I should know aboutfluvoxamine maleate extended-release capsules?
Fluvoxamine maleate extended-release capsules are the same kind of medicine as those used to treat depression. These medicines may cause serious side effects, including:
1. Suicidal thoughts or actions:
- Fluvoxamine maleate extended-release capsules and other antidepressant medicines may increase suicidal thoughts or actionsin some children, teenagers, or young adults within the first few months of treatment or when the dose is changed.
- Depression or other serious mental illnesses are the most important causes of suicidal thoughts or actions.
- Watch for these changes and call your healthcare provider right away if you notice:
- New or sudden changes in mood, behavior, actions, thoughts, or feelings, especially if severe.
- Pay particular attention to such changes when fluvoxamine maleate extended-release capsules are started or when the dose is changed.
Keep all follow-up visits with your healthcare provider and call between visits if you are worried about symptoms.
Call your healthcare provider right away if you have any of the following symptoms, or call 911 in an emergency, especially if they are new, worse, or worry you:
- attempts to commit suicide
- acting on dangerous impulses
- acting aggressive or violent
- thoughts about suicide or dying
- new or worse depression
- new or worse anxiety or panic attacks
- feeling agitated, restless, angry or irritable
- trouble sleeping
- an increase in activity or talking more than what is normal for you
- other unusual changes in behavior or mood
Call your healthcare provider right away if you have any of the following symptoms, or call 911 in an emergency.Fluvoxamine maleate extended-release capsulesmay be associated with these serious side effects:
2. Serotonin Syndrome. This condition can be life-threatening and may include:
- agitation, hallucinations, coma or other changes in mental status
- coordination problems or muscle twitching (overactive reflexes)
- racing heartbeat, high or low blood pressure
- sweating or fever
- nausea, vomiting, or diarrhea
- muscle rigidity
3.Visual Problems
- eye pain
- changes in vision
- swelling or redness in or around the eye
Only some people are at risk for these problems. You may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are.
4. Severe allergic reactions:
- trouble breathing
- swelling of the face, tongue, eyes, or mouth
- rash, itchy welts (hives) or blisters, alone or with fever or joint pain
5. Abnormal bleeding:Fluvoxamine maleate extended-release capsules and other antidepressant medicines may increase your risk of bleeding or bruising, especially if you take the blood thinner warfarin (Coumadin ®, Jantoven ®), a non-steroidal anti-inflammatory drug (NSAIDs, like ibuprofen or naproxen), or aspirin.
6. Seizures or convulsions
7. Manic episodes:
- greatly increased energy
- severe trouble sleeping
- racing thoughts
- reckless behavior
- unusually grand ideas
- excessive happiness or irritability
- talking more or faster than usual
8. Changes in appetite or weight.Children and adolescents should have height and weight monitored during treatment.
9. Low salt (sodium) levels in the blood. Elderly people may be at greater risk for this. Symptoms may include:
- headache
- weakness or feeling unsteady
- confusion, problems concentrating or thinking, or memory problems
10. Sexual problems (dysfunction).Taking selective serotonin reuptake inhibitors (SSRIs), including fluvoxamine maleate extended-release capsules, may cause sexual problems.
- Symptoms in males may include:
- Delayed ejaculation or inability to have an ejaculation
- Decreased sex drive
- Problems getting or keeping an erection
- Symptoms in females may include:
- Decreased sex drive
- Delayed orgasm or inability to have an orgasm
Talk to your healthcare provider if you develop any changes in your sexual function or if you have any questions or concerns about sexual problems during treatment with fluvoxamine maleate extended-release capsules. There may be treatments your healthcare provider can suggest.
Do not stop takingfluvoxamine maleate extended-release capsules without first talking to your healthcare provider.Stopping fluvoxamine maleate extended-release capsules may cause serious symptoms, including:
- anxiety, irritability, high or low mood, feeling restless or changes in sleep habits
- headache, sweating, nausea, dizziness
- electric shock-like sensations, shaking, confusion
What arefluvoxamine maleate extended-release capsules?
Fluvoxamine maleate extended-release capsules are prescription medicine used to treat obsessive compulsive disorder (OCD). It is the same kind of drug that is used to treat depression. It is important to talk with your healthcare provider about the risks of treating OCD and also the risks of not treating it. You should discuss all treatment choices with your healthcare provider.
Talk to your healthcare provider if you do not think that your condition is getting better with fluvoxamine maleate extended-release capsules treatment.
Who should not takefluvoxamine maleate extended-release capsules?
Do not take fluvoxamine maleate extended-release capsules if you:
- are allergic to fluvoxamine maleate or any of the ingredients in fluvoxamine maleate extended-release capsules. See the end of this Medication Guide for a complete list of ingredients in fluvoxamine maleate extended-release capsules.
- take a Monoamine Oxidase Inhibitor (MAOI). Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including the antibiotic linezolid.
- Do not take an MAOI within 2 weeks of stopping fluvoxamine maleate extended-release capsules unless directed to do so by your physician.
- Do not start fluvoxamine maleate extended-release capsules if you stopped taking an MAOI in the last 2 weeks unless directed to do so by your physician. People who takefluvoxamine maleate extended-release capsules close in time to an MAOI may have serious or even life-threatening side effects. Get medical help right away if you have any of these symptoms:
- high fever
- uncontrolled muscle spasms
- stiff muscles
- rapid changes in heart rate or blood pressure
- confusion
- loss of consciousness (pass out)
- take Mellaril ®(thioridazine). Mellaril ®should not be taken with fluvoxamine maleate extended-release capsules because this can cause serious heart rhythm problems or sudden death.
- take Zanaflex ®(tizanidine) because fluvoxamine maleate extended-release capsules can increase the amount of Zanaflex in your body, which could increase its actions and side effects. This could include causing drowsiness and a drop in blood pressure and affecting how well you do things that require alertness.
- take the antipsychotic medicine Orap ®(pimozide) because this can cause serious heart problems.
- take Lotronex ®(alosetron) because fluvoxamine maleate extended-release capsules can increase the amount of Lotronex in your body, which could increase its actions and side effects.
- take Rozerem ®(ramelteon) because fluvoxamine maleate extended-release capsules can increase the amount of Rozerem in your body, which could increase its actions and side effects.
What should I tell my healthcare provider before takingfluvoxamine maleate extended-release capsules? Ask if you are not sure.
Before starting fluvoxamine maleate extended-release capsules, tell your healthcare provider if you:
- are taking certain drugs such as:
- Clozaril ®(clozapine): used to treat schizophrenia
- Mexitil ®(mexiletine): used to treat problems with heart rhythm
- Triptans: used to treat migraine headache
- Medicines used to treat mood, anxiety, psychotic or thought disorders, including tricyclics, lithium, SSRIs, SNRIs, or antipsychotics
- Tramadol: used to reduce pain
- Meperidine
- Methadone
- or other opioids
- Benzodiazepines: used to reduce anxiety, stress, emotional upset, or seizures; helps you sleep; helps with alcohol withdrawal; reduces restlessness; and relaxes muscles
- Methadone: used to relieve pain or to help with addiction
- Theophylline: used to treat swollen air passages in your lungs, to relax the muscles in your chest to ease shortness of breath, often to treat asthma
- Warfarin and other drugs that affect how your blood clots
- Diuretics to treat high blood pressure, congestive heart failure, or swelling
- Over-the-counter supplements such as tryptophan or St. John’s Wort
- have liver problems
- have kidney problems
- have heart problems
- have or had seizures or convulsions
- have bipolar disorder or mania
- have low sodium levels in your blood
- have a history of a stroke
- have high blood pressure
- have or had bleeding problems
- are pregnant or plan to become pregnant. It is not known if fluvoxamine maleate extended-release capsules will harm your unborn baby. Talk to your healthcare provider about the benefits and risks of treating obsessive compulsive disorder (OCD) during pregnancy.
- are breastfeeding or plan to breastfeed. Some fluvoxamine maleate may pass into your breast milk. Talk to your healthcare provider about the best way to feed your baby while taking fluvoxamine maleate extended-release capsules.
Tell your healthcare provider about all the medicines that you take,including prescription and non-prescription medicines, vitamins, and herbal supplements. Fluvoxamine maleate extended-release capsules and some medicines may interact with each other, may not work as well, or may cause serious side effects.
Your healthcare provider or pharmacist can tell you if it is safe to take fluvoxamine maleate extended-release capsules with your other medicines. Do not start or stop any medicine while taking fluvoxamine maleate extended-release capsules without talking to your healthcare provider first.
If you take fluvoxamine maleate extended-release capsules, you should not take any other medicines that contain fluvoxamine maleate including: Fluvoxamine Maleate Immediate-Release Tablets.
How should I takefluvoxamine maleate extended-release capsules?
- Take fluvoxamine maleate extended-release capsules at night exactly as prescribed. Your healthcare provider may need to change the dose of fluvoxamine maleate extended-release capsules until it is the right dose for you.
- Fluvoxamine maleate extended-release capsules may be taken with or without food.
- If you miss a dose of fluvoxamine maleate extended-release capsules, take the missed dose as soon as you remember. If it is almost time for the next dose, skip the missed dose and take your next dose at the regular time. Do not take two doses of fluvoxamine maleate extended-release capsules at the same time.
- If you take too much fluvoxamine maleate extended-release capsules, call your healthcare provider or poison control center right away, or get emergency treatment.
What should I avoid while takingfluvoxamine maleate extended-release capsules?
Fluvoxamine maleate extended-release capsules can cause sleepiness or may affect your ability to make decisions, think clearly, or react quickly. You should not drive, operate heavy machinery, or do other dangerous activities until you know how fluvoxamine maleate extended-release capsules affect you. Do not drink alcohol while using fluvoxamine maleate extended-release capsules.
What are the possible side effects offluvoxamine maleate extended-release capsules?
Fluvoxamine maleate extended-release capsules may cause serious side effects, including all of those described in the section entitled “What is the most important information I should know about fluvoxamine maleate extended-release capsules?”
Common possible side effects in people who take fluvoxamine include:
- Nausea
- Sleepiness
- Weakness
- Dizziness
- Feeling anxious
- Trouble sleeping
- Sexual problems
- Sweating
- Shaking
- Not feeling hungry
- Dry mouth
- Diarrhea
- Muscle pain
- Sore throat
- Throwing up
- Upset stomach
- Yawning
Other side effects in children and adolescents taking fluvoxamine include:
- abnormal increase in muscle movement or agitation
- depression
- heavy menstrual periods
- flatulence (gas)
- rash
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of fluvoxamine maleate extended-release capsules. For more information, ask your healthcare provider or pharmacist.
CALL YOUR DOCTOR FOR MEDICAL ADVICE ABOUT SIDE EFFECTS. YOU MAY REPORT SIDE EFFECTS TO FDA AT 1-800-FDA-1088.
How should I storefluvoxamine maleate extended-release capsules?
- Store fluvoxamine maleate extended-release capsules at 59° to 86°F (15° to 30°C).
- Keep fluvoxamine maleate extended-release capsules away from high temperatures (above 86°F or 30°C) and high humidity (dampness).
- Keep the fluvoxamine maleate extended-release capsules bottle closed tightly.
- Fluvoxamine maleate extended-release capsules comes in a child-resistant package.
Keepfluvoxamine maleate extended-release capsules and all medicines out of the reach of children.
General information aboutfluvoxamine maleate extended-release capsules
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use fluvoxamine maleate extended-release capsules for a condition for which it was not prescribed. Do not give fluvoxamine maleate extended-release capsules to other people, even if they have the same condition. It may harm them.
This Medication Guide summarizes the most important information about fluvoxamine maleate extended-release capsules. If you would like more information, talk with your healthcare provider. You may ask your healthcare provider or pharmacist for information about fluvoxamine maleate extended-release capsules that is written for healthcare professionals.
For more information about fluvoxamine maleate extended-release capsules, call 1-888-235-BION or 1-888-235-2466.
What are the ingredients influvoxamine maleate extended-release capsules?
Active ingredient:fluvoxamine maleate, USP
Inactive ingredients: D&C Red 28, ethylcellulose, FD&C Blue 1, gelatin, hydroxypropyl cellulose, hypromellose, povidone, sugar spheres, triethyl citrate, and titanium dioxide. The capsules are printed with edible ink containing black iron oxide, potassium hydroxide, propylene glycol and shellac.
Distributed by:
Bionpharma Inc.
Princeton, NJ 08540MADE IN INDIA
Revised: October 2023
FDA-06
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Brands listed are the trademarks of their respective owners.
948026815
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FLUVOXAMINE MALEATE
fluvoxamine maleate capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69452-182 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUVOXAMINE MALEATE (UNII: 5LGN83G74V) (FLUVOXAMINE - UNII:O4L1XPO44W) FLUVOXAMINE MALEATE 100 mg Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) D&C RED NO. 28 (UNII: 767IP0Y5NH) ETHYLCELLULOSE (7 MPA.S) (UNII: H3UP11403C) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) POVIDONE K30 (UNII: U725QWY32X) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) Product Characteristics Color purple (Opaque Cap) , white (Opaque Body) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code FL;100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69452-182-13 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/01/2020 2 NDC:69452-182-32 1000 in 1 BOTTLE; Type 0: Not a Combination Product 12/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212182 12/01/2020 FLUVOXAMINE MALEATE
fluvoxamine maleate capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69452-183 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUVOXAMINE MALEATE (UNII: 5LGN83G74V) (FLUVOXAMINE - UNII:O4L1XPO44W) FLUVOXAMINE MALEATE 150 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 28 (UNII: 767IP0Y5NH) ETHYLCELLULOSE (7 MPA.S) (UNII: H3UP11403C) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) POVIDONE K30 (UNII: U725QWY32X) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) Product Characteristics Color purple (Opaque Cap) , white (Opaque Body) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code FL;150 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69452-183-13 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/01/2020 2 NDC:69452-183-32 1000 in 1 BOTTLE; Type 0: Not a Combination Product 07/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212182 07/01/2020 Labeler - Bionpharma Inc. (079637826) Registrant - Bionpharma Inc. (079637826) Establishment Name Address ID/FEI Business Operations OrBion Pharmaceuticals Private Limited 854403569 manufacture(69452-182, 69452-183)