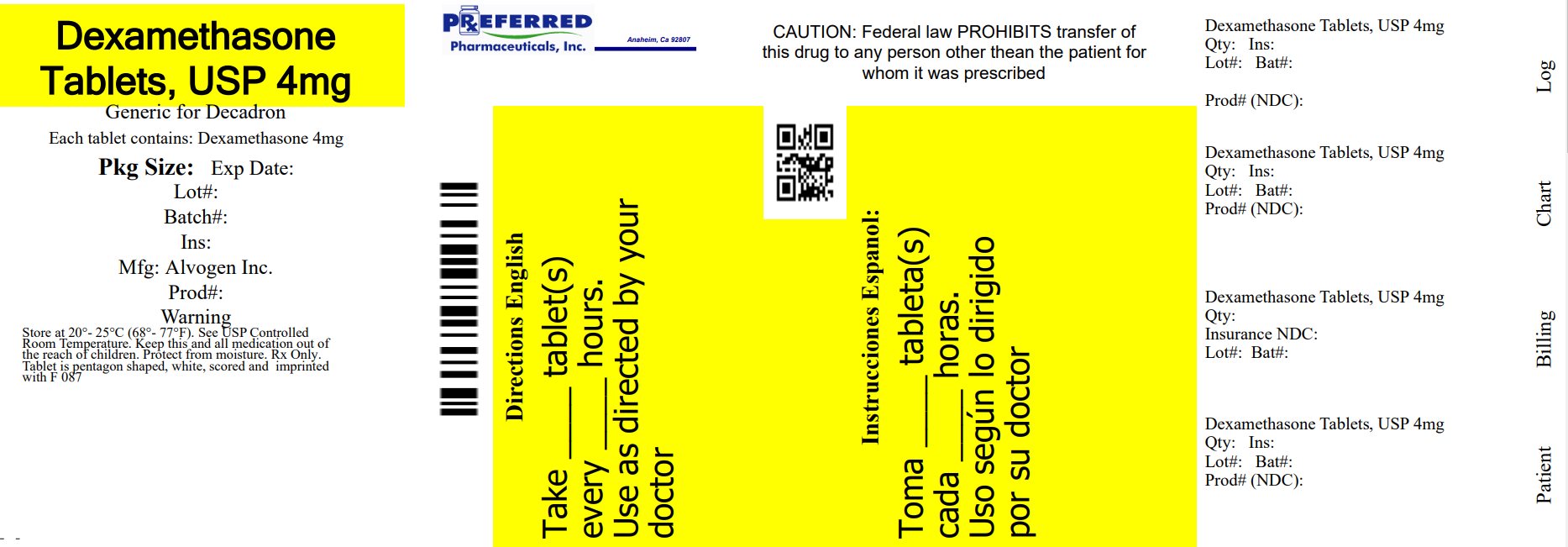
Label: DEXAMETHASONE tablet
- NDC Code(s): 68788-8765-1, 68788-8765-3, 68788-8765-5, 68788-8765-6
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 47781-914
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
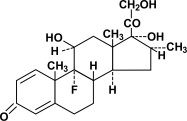
DESCRIPTION Dexamethasone Tablets 0.5 mg, 0.75 mg, 4 mg and 6 mg are for oral administration. Each tablet contains 0.5 mg, 0.75 mg, 4 mg or 6 mg of dexamethasone. Dexamethasone, a synthetic adrenocortical ...
-
CLINICAL PHARMACOLOGY Glucocorticoids, naturally occurring and synthetic, are adrenocortical steroids that are readily absorbed from the gastrointestinal tract. Glucocorticoids cause varied metabolic effects. In ...
-
INDICATIONS AND USAGE Allergic States - Control of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment in asthma, atopic dermatitis, contact dermatitis, drug ...
-
CONTRAINDICATIONS Systemic fungal infections (see Error! Hyperlink reference not valid.: Fungal Infections). Dexamethasone tablets are contraindicated in patients who are hypersensitive to any components of this ...
-
WARNINGS General: Rare instances of anaphylactoid reactions have occurred in patients receiving corticosteroid therapy (see ADVERSE REACTIONS). Increased dosage of rapidly acting corticosteroids is ...
-
PRECAUTIONS General: The lowest possible dose of corticosteroids should be used to control the condition under treatment. When reduction in dosage is possible, the reduction should be gradual. Since ...
-
ADVERSE REACTIONS (listed alphabetically, under each subsection) The following adverse reactions have been reported with dexamethasone or other corticosteroids: Allergic Reactions: Anaphylactoid reaction ...
-
OVERDOSAGE Treatment of overdosage is by supportive and symptomatic therapy. In the case of acute overdosage, according to the patient's condition, supportive therapy may include gastric lavage or emesis ...
-
DOSAGE AND ADMINISTRATION For Oral Administration: The initial dose varies from 0.75 to 9 mg a day depending on the disease being treated. IT SHOULD BE EMPHASIZED THAT DOSAGE REQUIREMENTS ARE VARIABLE AND MUST BE ...
-
HOW SUPPLIED Dexamethasone tablets are available as: 4 mg tablets scored (white), debossed “F 087” and supplied in: Bottles of 6 Tablets NDC 68788-8765-6 - Bottles of 12 Tablets NDC 68788-8765-12 - Bottles of 15 ...
-
Principal Display Panel - 4 mg TabletsNDC 68788-8765 - Dexamethasone Tablets, USP - 4 mg - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information