Label: OMISIRGE- omidubicel-onlv kit
- NDC Code(s): 73441-100-01, 73441-200-01, 73441-300-01, 73441-400-01, view more
- Packager: Gamida Cell Inc.
- Category: CELLULAR THERAPY
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated January 10, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use OMISIRGE safely and effectively. See full prescribing information for OMISIRGE. OMISIRGE® (omidubicel-onlv) Suspension for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: INFUSION REACTIONS, GRAFT VERSUS HOST DISEASE, ENGRAFTMENT SYNDROME, and GRAFT FAILURE
- Infusion reactions: Infusion reactions may be fatal. Monitor patients during infusion and discontinue for severe reactions. Use is contraindicated in patients with known allergy to dimethyl sulfoxide (DMSO), Dextran 40, gentamicin, human serum albumin, or bovine material [see Contraindications (4), Warnings and Precautions (5.1, 5.2)].
- Graft-vs-Host Disease (GvHD): GvHD may be fatal. Administration of immunosuppressive therapy may decrease the risk of GvHD [see Warnings and Precautions (5.3)].
- Engraftment syndrome: Engraftment syndrome may be fatal. Treat engraftment syndrome promptly with corticosteroids [see Warnings and Precautions (5.4)].
- Graft failure: Graft failure may be fatal. Monitor patients for laboratory evidence of hematopoietic recovery [see Warnings and Precautions (5.5)].
-
1 INDICATIONS AND USAGEOMISIRGE is a nicotinamide modified allogeneic hematopoietic progenitor cell therapy derived from cord blood indicated for use in adults and pediatric patients 12 years and older with hematologic ...
-
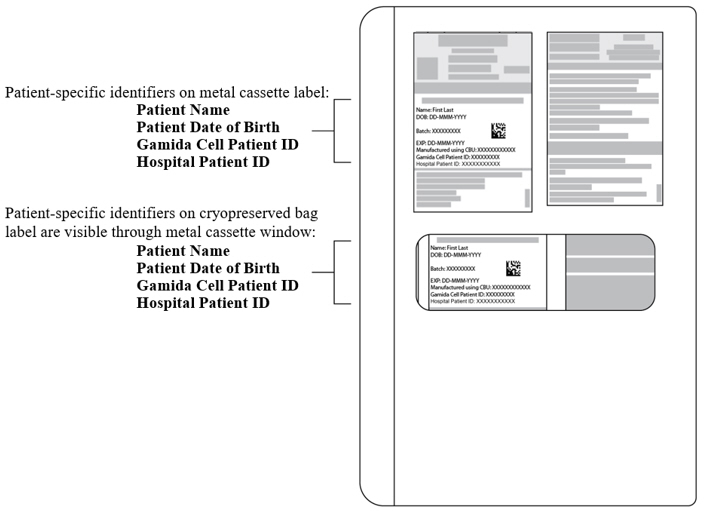
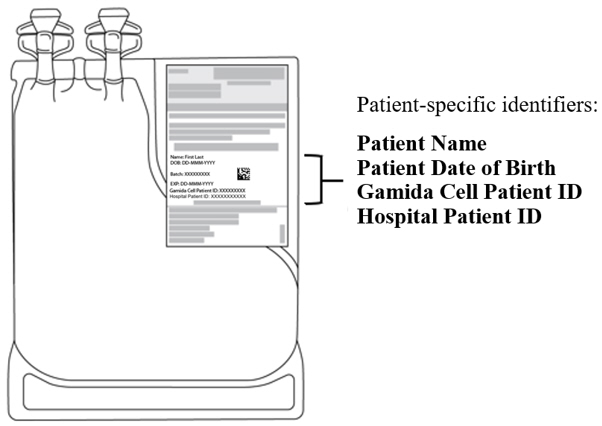
2 DOSAGE AND ADMINISTRATIONFor intravenous use only. Do not irradiate. Do NOT use a leukodepleting filter. Verify patient's identity upon receipt. Do NOT open the metal cassettes until time of thaw. Verify patient's ...
-
3 DOSAGE FORMS AND STRENGTHSOMISIRGE is a cell suspension for intravenous infusion. A single dose of OMISIRGE consists of: a Cultured Fraction: At the time of cryopreservation, the CF contains a minimum of 8.0 × 108 total ...
-
4 CONTRAINDICATIONSOMISIRGE is contraindicated in patients with known hypersensitivity to dimethyl sulfoxide (DMSO), Dextran 40, gentamicin, human serum albumin, or bovine products.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Allergic reactions may occur with the infusion of OMISIRGE. Reactions include bronchospasm, wheezing, angioedema, pruritis and hives. Serious hypersensitivity ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse events are discussed in greater detail elsewhere in the labeling: Hypersensitivity Reactions [See Warnings and Precautions (5.1)] Infusion Reactions ...
-
7 DRUG INTERACTIONSNo studies of drug interaction have been performed with OMISIRGE.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data with OMISIRGE use in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with OMISIRGE to ...
-
11 DESCRIPTIONOMISIRGE (omidubicel-onlv) is a cryopreserved nicotinamide modified allogeneic hematopoietic progenitor cell therapy derived from cord blood consisting of 2 cell fractions; a Cultured Fraction ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - OMISIRGE is a nicotinamide (NAM) modified allogeneic hematopoietic progenitor cell therapy derived from cord blood used as an allogeneic stem cell donor source ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No in vivo carcinogenicity, mutagenicity and fertility studies were conducted to evaluate the effects of OMISIRGE.
-
14 CLINICAL STUDIESOMISIRGE was evaluated in Study P0501 (NCT02730299), an open-label, multicenter, randomized study of OMISIRGE transplantation or UCB transplantation following myeloablative conditioning in ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGOMISIRGE (NDC 73441-800-04) is shipped in two shipping containers, a liquid nitrogen dry vapor shipper at ≤ -150℃, containing the two cryopreserved cell fractions (CF NDC 73441-100-01 and NF NDC ...
-
17 PATIENT COUNSELING INFORMATIONDiscuss the following with the patient receiving OMISIRGE: The recommended course of therapy for OMISIRGE is a single dose for infusion, which is provided by the manufacturer as 2 separate ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Gamida Cell Ltd., Kiryat Gat 8258412, Israel - U.S. License number 2223 - Distributed by: Gamida Cell Inc., Naples, FL 34102 - OMISIRGE® is a registered trademark of Gamida Cell Inc. ...
-
PRINCIPAL DISPLAY PANEL - 20 mL and 10 mL Shipping LabelNDC 73441-100-01 - NDC 73441-200-01 - Gamida Cell Patient ID: Hospital Patient ID: Batch: Expiry Date: DD-MMM-CCYY - omidubicel-onlv - OMISIRGE® Rx Only - Container 1 of 2 - Cultured Fraction - Non-cultured ...
-
PRINCIPAL DISPLAY PANEL - 80 mL and 40 mL Shipping LabelNDC 73441-300-01 - NDC 73441-400-01 - Gamida Cell Patient ID: Hospital Patient ID: Batch: Expiry Date: DD-MMM-CCYY - omidubicel-onlv - OMISIRGE® Rx Only - Container 2 of 2 - Infusion Solution for - Cultured ...
-
INGREDIENTS AND APPEARANCEProduct Information