Label: BAEKCHO-DS- licorice ext syrup
- NDC Code(s): 73442-0007-1
- Packager: I World Pharmaceutical Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
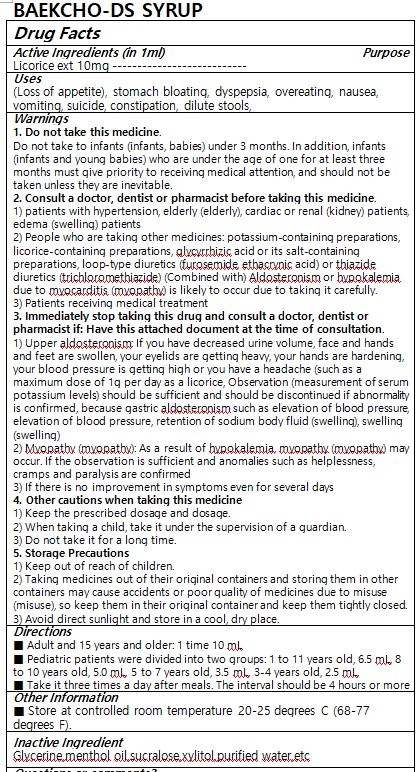
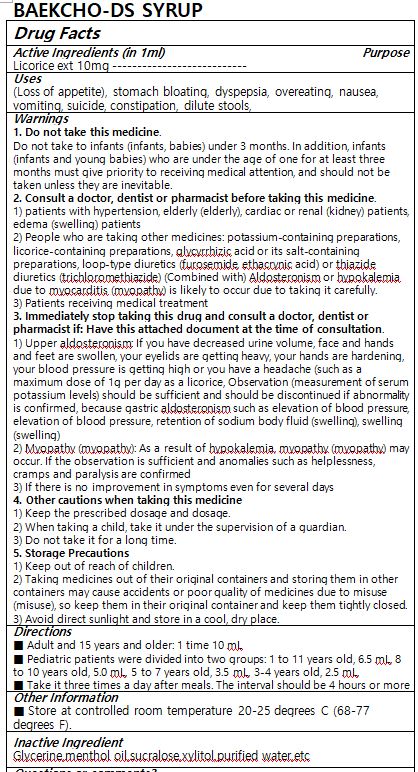
WARNINGS
1. Do not take this medicine.
Do not take to infants (infants, babies) under 3 months. In addition, infants (infants and young babies) who are under the age of one for at least three months must give priority to receiving medical attention, and should not be taken unless they are inevitable.2. Consult a doctor, dentist or pharmacist before taking this medicine.
1) patients with hypertension, elderly (elderly), cardiac or renal (kidney) patients, edema (swelling) patients
2) People who are taking other medicines: potassium-containing preparations, licorice-containing preparations, glycyrrhizic acid or its salt-containing preparations, loop-type diuretics (furosemide, ethacrynic acid) or thiazide diuretics (trichloromethiazide) (Combined with) Aldosteronism or hypokalemia due to myocarditis (myopathy) is likely to occur due to taking it carefully.
3) Patients receiving medical treatment3. Immediately stop taking this drug and consult a doctor, dentist or pharmacist if: Have this attached document at the time of consultation.
1) Upper aldosteronism: If you have decreased urine volume, face and hands and feet are swollen, your eyelids are getting heavy, your hands are hardening, your blood pressure is getting high or you have a headache (such as a maximum dose of 1g per day as a licorice, Observation (measurement of serum potassium levels) should be sufficient and should be discontinued if abnormality is confirmed, because gastric aldosteronism such as elevation of blood pressure, elevation of blood pressure, retention of sodium body fluid (swelling), swelling (swelling)
2) Myopathy (myopathy): As a result of hypokalemia, myopathy (myopathy) may occur. If the observation is sufficient and anomalies such as helplessness, cramps and paralysis are confirmed
3) If there is no improvement in symptoms even for several days4. Other cautions when taking this medicine
1) Keep the prescribed dosage and dosage.
2) When taking a child, take it under the supervision of a guardian.
3) Do not take it for a long time.5. Storage Precautions
1) Keep out of reach of children.
2) Taking medicines out of their original containers and storing them in other containers may cause accidents or poor quality of medicines due to misuse (misuse), so keep them in their original container and keep them tightly closed.
3) Avoid direct sunlight and store in a cool, dry place. - DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BAEKCHO-DS
licorice ext syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73442-0007 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LICORICE (UNII: 61ZBX54883) (LICORICE - UNII:61ZBX54883) LICORICE 10 g in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) SUCRALOSE (UNII: 96K6UQ3ZD4) MENTHOL (UNII: L7T10EIP3A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73442-0007-1 100 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 03/29/2019 12/11/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/23/2019 12/11/2024 Labeler - I World Pharmaceutical Co., Ltd. (688222857) Registrant - I World Pharmaceutical Co., Ltd. (688222857) Establishment Name Address ID/FEI Business Operations I World Pharmaceutical Co., Ltd. 688222857 manufacture(73442-0007)