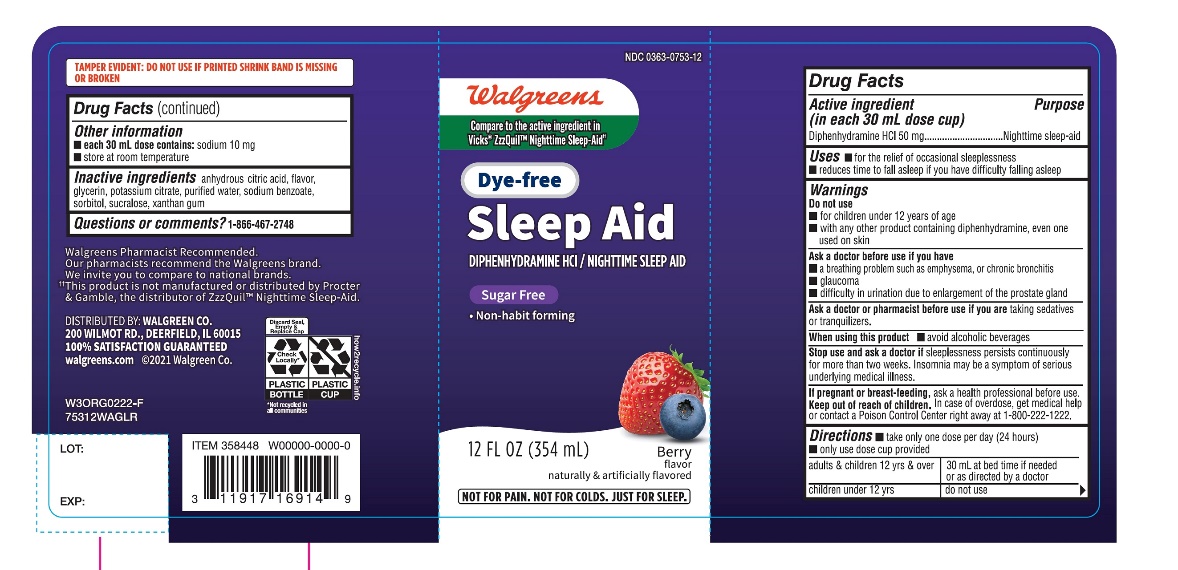
Label: DYE FREE WAL SLEEP Z- diphenhydramine hydrochloride liquid
- NDC Code(s): 0363-0753-12
- Packager: WALGREEN CO.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 30 mL)
- Purpose
- Uses
-
Warnings
Do not use
- •
- for children under 12 years of age
- •
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- •
- a breathing problem such as emphysema, or chronic bronchitis
- •
- glaucoma
- •
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
-
PRINCIPAL DISPLAY PANEL
Walgreens
Compare to Vicks® ZzzQuil® Nighttime Sleep-Aid active ingredient ††
NDC 0363-0753-12
Dye –Free
Wal-Sleep Z®
Diphenhydramine HCl / Nighttime Sleep Aid
DYE FREE
SUGAR FREE
- •
- Non – habit forming
BERRY FLAVOR
12 FL OZ (354 mL)
NOT FOR TREATING COLD OR FLU,SEE WARNINGS.
FAILURE TO FOLLOW THESE WARNINGS COULD RESULT IN SERIOUS CONSEQUENCES.
TAMPER EVIDENT: DO NOT USE IF PRINTED SHRINK BAND IS MISSING OR BROKEN
Walgreens Pharmacist Recommended
Walgreens Pharmacist Survey
†† This product is not manufactured or distributed by Procter & Gamble, the distributor of ZzzQuil™ Nighttime Sleep-Aid.
DISTRIBUTED BY: WALGREEN CO.
200 WILMONT RD, DEERFIELD, IL 60015
100% SATISFACTION GUARANTEED
Walgreens.com ©2018 Walgreen Co.
-
INGREDIENTS AND APPEARANCE
DYE FREE WAL SLEEP Z
diphenhydramine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-0753 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 50 mg in 30 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM CITRATE (UNII: EE90ONI6FF) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-0753-12 354 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/24/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/24/2018 Labeler - WALGREEN CO. (008965063)