Label: INFANTS GAS RELIEF DROPS- simethicone emulsion
- NDC Code(s): 49035-709-01, 49035-709-30
- Packager: Wal-Mart Stores,Inc.,
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
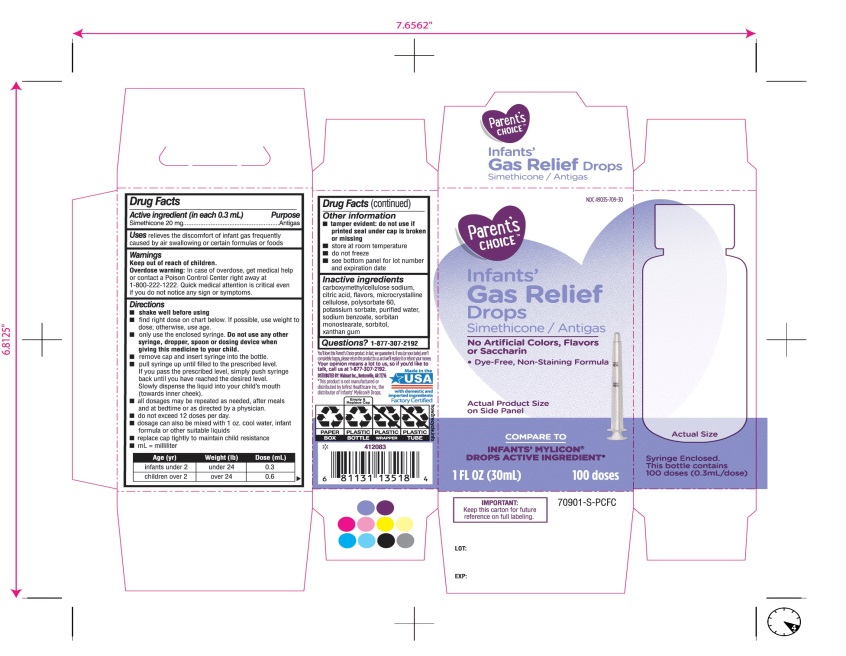
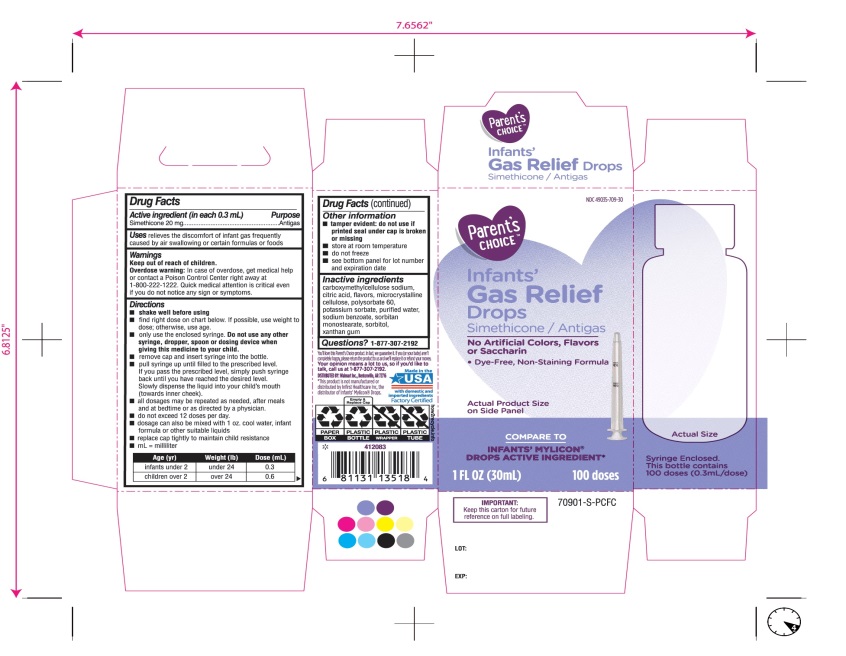
- Active ingredient (in each 0.3 mL)
- Purpose
- Uses
- Warnings
-
Directions
- ▪
- shake well before using
- ▪
- find right dose on chart below. if possible, use weight to dose; otherwise use age.
- ▪
- Only use the enclosed syringe. Do not use any other syringe, dropper, spoon or dosing device when giving this medicine to your child.
- ▪
- remove cap and insert syringe into the prescribed level.
- ▪
- pull syringe up until filled to the prescribed level. If you pass the prescribed level, simply push syringe back until you have reached the desired level.
slowly dispense the liquid into your child’s mouth (towards inner chick.
- ▪
- all dosages may be repeated as needed, after meals and at bedtime or as directed by a physician.
- ▪
- do not exceed 12 doses per day.
- ▪
- dosage can also be mixed with 1 oz. cool water, infant formula or other suitable liquids
- ▪
- replace cap tightly to maintain child resistance
- ▪
- mL= milliliter
Age (yr)
Weight (lb)
Dose( mL)
infants under 2
under 24
0.3
children over 2
over 24
0.6
- Other information
- Inactive ingredients
-
Principal Display Panel
COMPARE TO INFANT’S MYLICON® DROPS ACTIVE INGREDIENT*
Parent’s CHOICE™
Infants'
Gas Relief
Drops
Simethicone /Antigas
No Artificial Colors, Flavors or Saccharin
Dye-Free, Non-Staining Formula
1 FL. OZ. (30 mL) 100 doses
DISTRIBUTED BY: Walmart, Bentonville, AR 72716
Made in USA with domestic and imported ingredients.
Factory certified.
Syringe Enclosed. This bottle contains 100 doses (0.3mL/dose)
* This product is not manufactured or distributed by Infirst Healthcare Inc., the distributor of Infants’ Mylicon® Drops.
- IMPORTANT: Keep this carton for future reference on full labeling
-
INGREDIENTS AND APPEARANCE
INFANTS GAS RELIEF DROPS
simethicone emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49035-709 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE, UNSPECIFIED (UNII: 92RU3N3Y1O) (DIMETHICONE, UNSPECIFIED - UNII:92RU3N3Y1O) DIMETHICONE, UNSPECIFIED 20 mg in 0.3 mL Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYSORBATE 60 (UNII: CAL22UVI4M) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) SORBITOL (UNII: 506T60A25R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color WHITE (white to off white, opaque) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49035-709-01 1 in 1 CARTON 10/16/2017 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:49035-709-30 1 in 1 CARTON 10/22/2019 2 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M002 10/16/2017 Labeler - Wal-Mart Stores,Inc., (051957769)