Label: GLYCOPYRROLATE injection
- NDC Code(s): 0641-6212-01, 0641-6212-10, 0641-6213-01, 0641-6213-10
- Packager: Hikma Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
DESCRIPTION
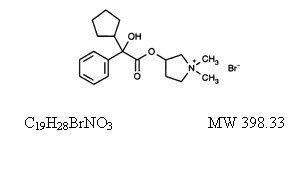
Glycopyrrolate Injection, USP is a synthetic anticholinergic agent. Each 1 mL contains: Glycopyrrolate, USP 0.2 mg - Water for Injection, USP q.s. pH adjusted, when necessary, with hydrochloric ...
-
CLINICAL PHARMACOLOGY
Glycopyrrolate, like other anticholinergic (antimuscarinic) agents, inhibits the action of acetylcholine on structures innervated by postganglionic cholinergic nerves and on smooth muscles that ...
-
INDICATIONS AND USAGE
In Anesthesia - Glycopyrrolate Injection is indicated for use as a preoperative antimuscarinic to reduce salivary, tracheobronchial, and pharyngeal secretions; to reduce the volume and free ...
-
CONTRAINDICATIONS
Known hypersensitivity to glycopyrrolate or any of its inactive ingredients. In addition, in the management of peptic ulcer patients, because of the longer duration of therapy, Glycopyrrolate ...
-
WARNINGS
This drug should be used with great caution, if at all, in patients with glaucoma. Glycopyrrolate Injection may produce drowsiness or blurred vision. The patient should be cautioned regarding ...
-
PRECAUTIONS
General - Investigate any tachycardia before giving Glycopyrrolate Injection since an increase in the heart rate may occur. Use with caution in patients with: coronary artery disease ...
-
ADVERSE REACTIONS
Anticholinergics, including Glycopyrrolate Injection, can produce certain effects, most of which are extensions of their pharmacologic actions. Adverse reactions may include xerostomia (dry ...
-
OVERDOSAGE
To combat peripheral anticholinergic effects, a quaternary ammonium anticholinesterase such as neostigmine methylsulfate (which does not cross the blood-brain barrier) may be given intravenously ...
-
DOSAGE AND ADMINISTRATION
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Glycopyrrolate Injection may be ...
-
HOW SUPPLIED
Glycopyrrolate Injection, USP, 0.2 mg per mL is available as a preservative-free, clear, colorless solution supplied in single-dose prefilled syringes as follows: 1 mL single-dose prefilled ...
-
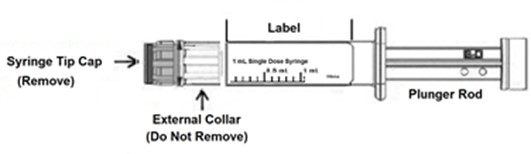
INSTRUCTIONS FOR USE
CAUTION: Glass syringes may malfunction, break or clog when connected to some Needleless Luer Access Devices (NLADs) and needles. The external collar must remain attached to the syringe (See ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Hikma Pharmaceuticals USA Inc. Berkeley Heights, NJ 07922 - 462-059-00 - Revised July 2024
-
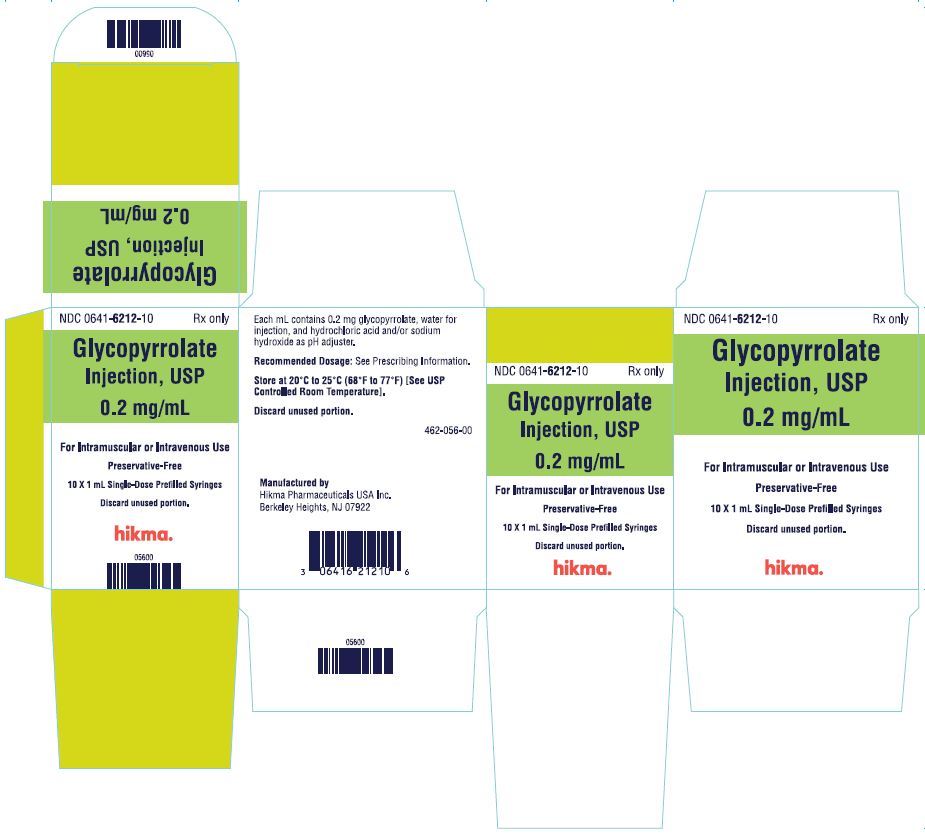
PRINCIPAL DISPLAY PANEL
NDC 0641-6212-01Rx only - 1 mL Single-Dose Prefilled Syringe - Glycopyrrolate Injection, USP - 0.2 mg/mL - For IM or IV Use - Preservative-Free. Discard unused portion. NDC 0641-6212-10 Rx ...
-
PRINCIPAL DISPLAY PANEL
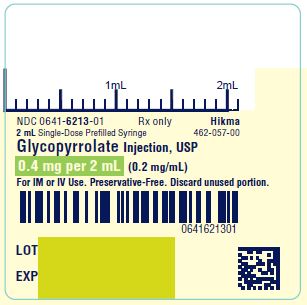
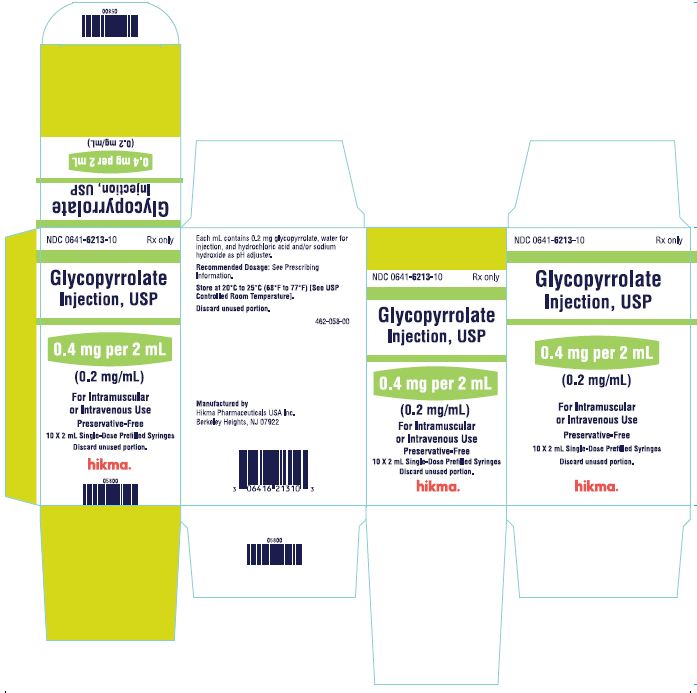
NDC 0641-6213-01Rx only - 2 mL Single-Dose Prefilled Syringe - Glycopyrrolate Injection, USP - 0.4 mg per 2 mL (0.2 mg/mL) For IM or IV Use. Preservative-Free. Discard unused portion. NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information