Label: BIVALIRUDIN injection, powder, lyophilized, for solution
- NDC Code(s): 67457-256-00, 67457-256-10
- Packager: Mylan Institutional LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 24, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BIVALIRUDIN FOR INJECTION safely and effectively. See full prescribing information for BIVALIRUDIN FOR INJECTION. BIVALIRUDIN for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEBivalirudin for injection is indicated for use as an anticoagulant for use in patients undergoing percutaneous coronary intervention (PCI) including patients with heparin-induced thrombocytopenia ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Bivalirudin for injection has been studied only in patients receiving concomitant aspirin. The recommended dose of bivalirudin for injection is an intravenous bolus dose ...
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 250 mg of bivalirudin, USP as a lyophilized powder in a single-dose vial for reconstitution. Each vial contains 250 mg of bivalirudin, USP equivalent to an average of 275 mg ...
-
4 CONTRAINDICATIONSBivalirudin for injection is contraindicated in patients with: • Active major bleeding; • Hypersensitivity (e.g., anaphylaxis) to bivalirudin for injection or its components [see Adverse ...
-
5 WARNINGS AND PRECAUTIONS5.1 Bleeding Events - Bivalirudin for injection increases the risk of bleeding [see Adverse Reactions (6.1)]. An unexplained fall in blood pressure or hematocrit should lead to serious ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSIn clinical trials in patients undergoing PCI/percutaneous transluminal coronary angioplasty (PTCA), co-administration of bivalirudin with heparin, warfarin, thrombolytics, or GPIs was associated ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no data available on use of bivalirudin in pregnant women to inform a drug-associated risk of adverse developmental outcomes. Reproduction studies in ...
-
10 OVERDOSAGECases of overdose of up to 10 times the recommended bolus or continuous infusion dose of bivalirudin have been reported in clinical trials and in postmarketing reports. A number of the reported ...
-
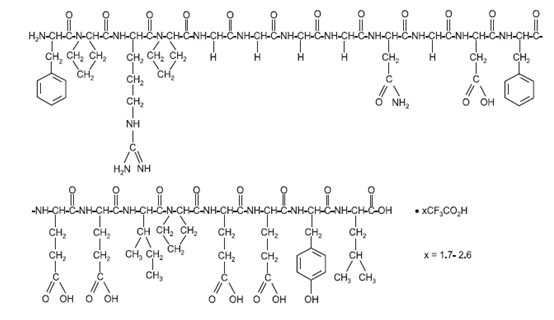
11 DESCRIPTIONBivalirudin for injection, USP contains bivalirudin, USP which is a specific and reversible direct thrombin inhibitor. Bivalirudin, USP is a synthetic, 20 amino acid peptide, with the chemical ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Bivalirudin directly inhibits thrombin by specifically binding both to the catalytic site and to the anion-binding exosite of circulating and clot-bound thrombin ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No long-term studies in animals have been performed to evaluate the carcinogenic potential of bivalirudin. Bivalirudin displayed no ...
-
14 CLINICAL STUDIESBivalirudin Angioplasty Trial (BAT) In the BAT studies, patients with unstable angina undergoing PCI were randomized 1:1 to a 1 mg/kg bolus of bivalirudin and then 2.5 mg/kg/h for four hours and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Bivalirudin for Injection, USP is supplied as a sterile, white lyophilized powder or cake in single-dose, glass vials. Each vial contains 250 mg of bivalirudin, USP ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to watch carefully for any signs of bleeding or bruising and to report these to their health care provider when they occur. Manufactured for: Mylan Institutional LLC - Morgantown ...
-
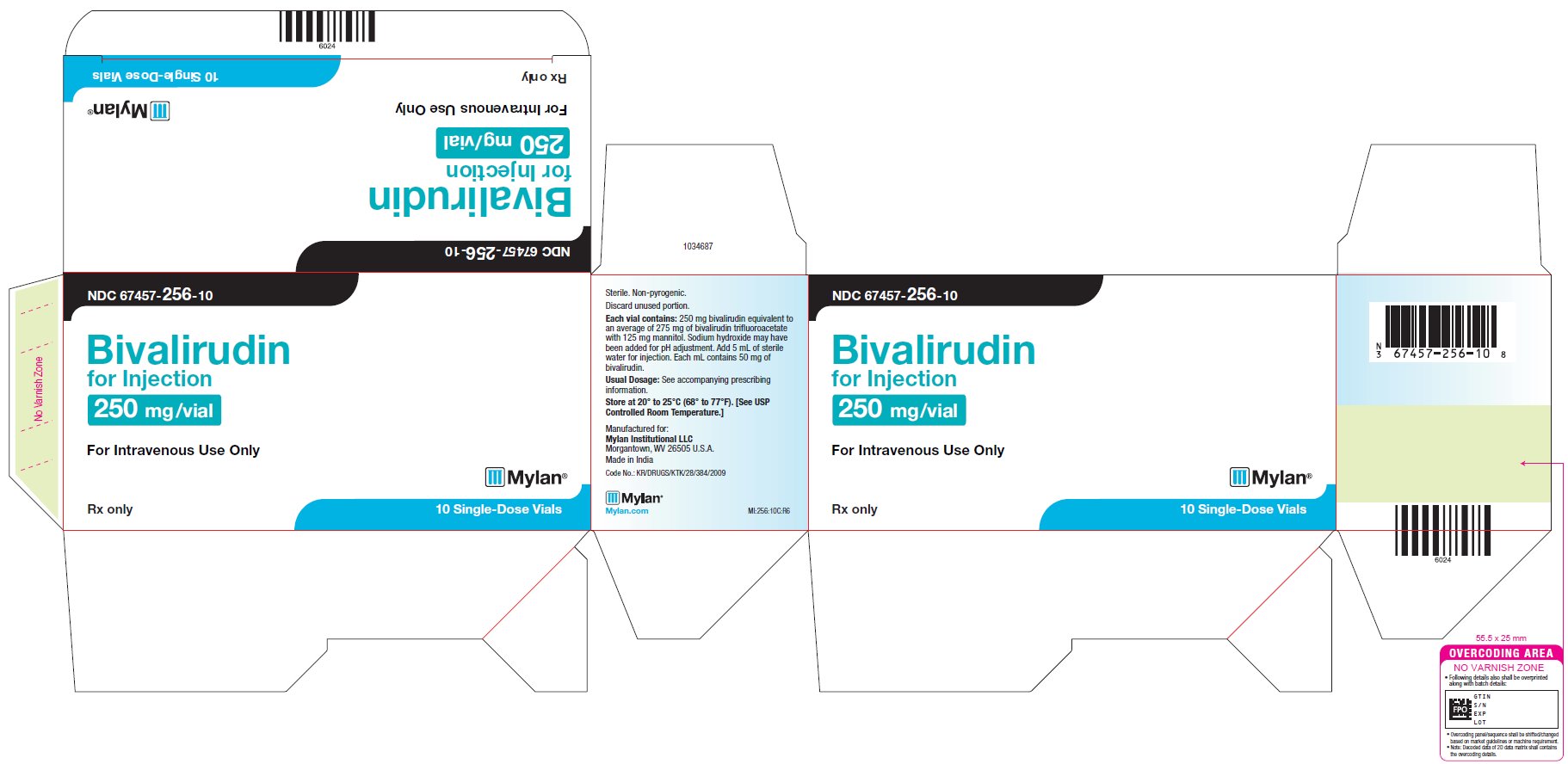
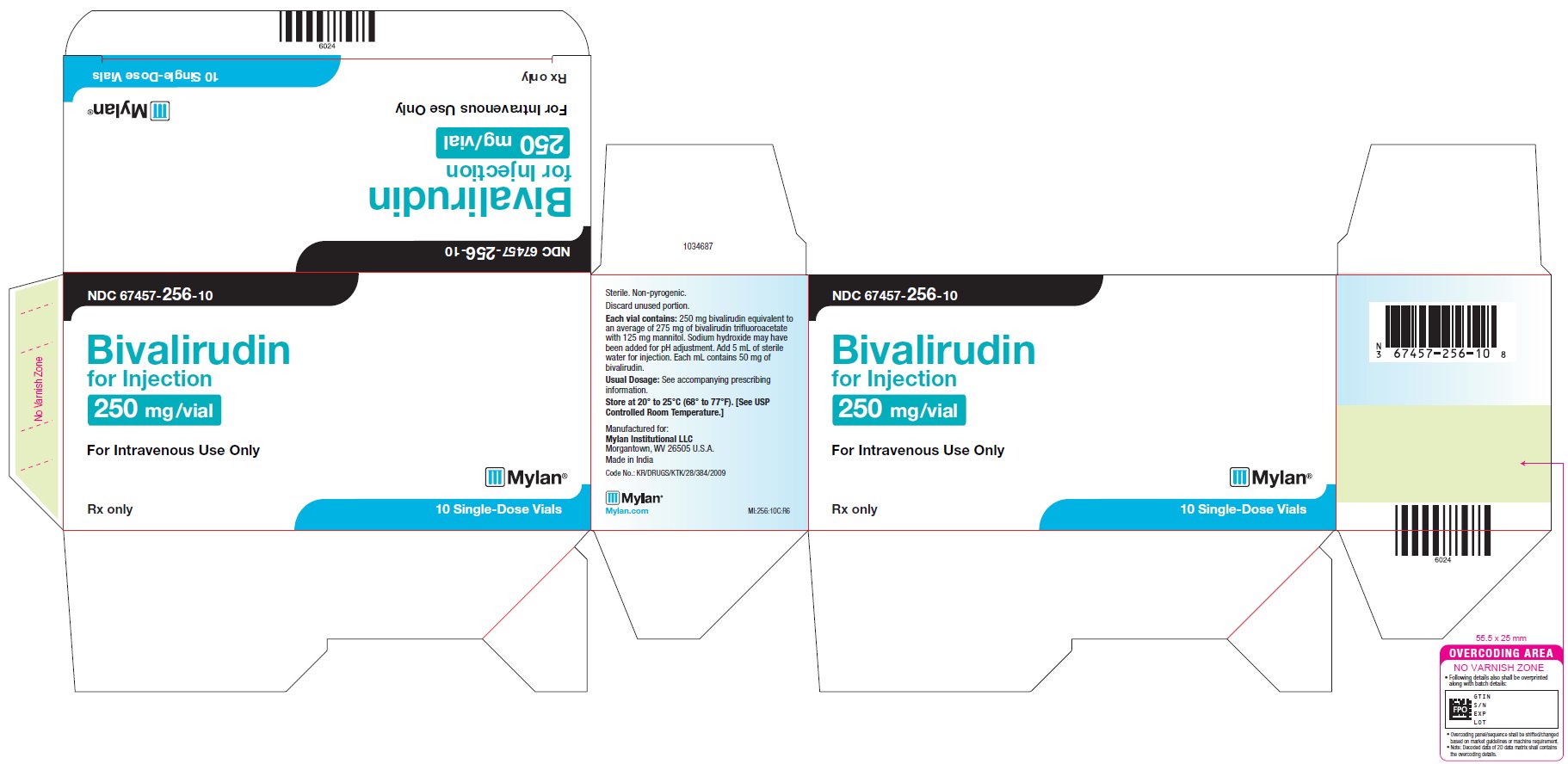
PRINCIPAL DISPLAY PANEL – 250 mg/Vial NDC 67457-256-10 - Bivalirudin - for Injection, USP - 250 mg /vial - For Intravenous Use Only - Rx only 10 Single-Dose Vials - Sterile. Non-pyrogenic. Discard unused portion. Each vial contains ...
-
INGREDIENTS AND APPEARANCEProduct Information