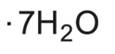Label: ZINC SULFATE- zinc sulfate injection, solution
- NDC Code(s): 70710-1876-1, 70710-1876-7, 70710-1877-1, 70710-1877-7, view more
- Packager: Zydus Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZINC SULFATE - INJECTION safely and effectively. See full prescribing information for ZINC SULFATE INJECTION. ZINC SULFATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZinc Sulfate Injection is indicated in adult and pediatric patients as a source of zinc for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Information - Zinc Sulfate Injection is supplied as a pharmacy bulk package for admixing use only. It is not for direct intravenous infusion. Prior to ...
-
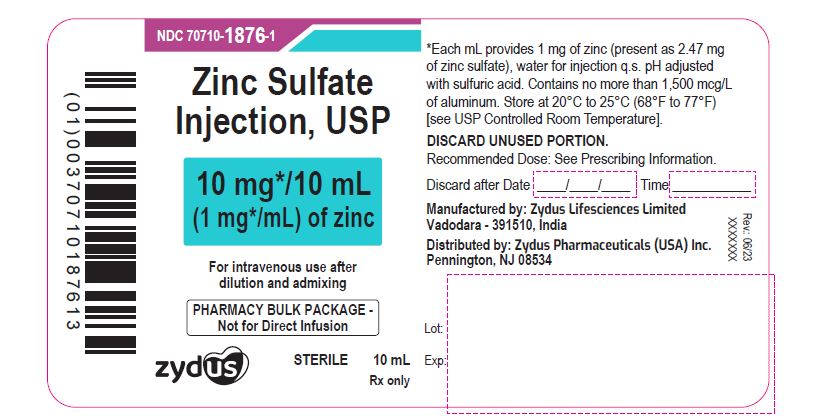
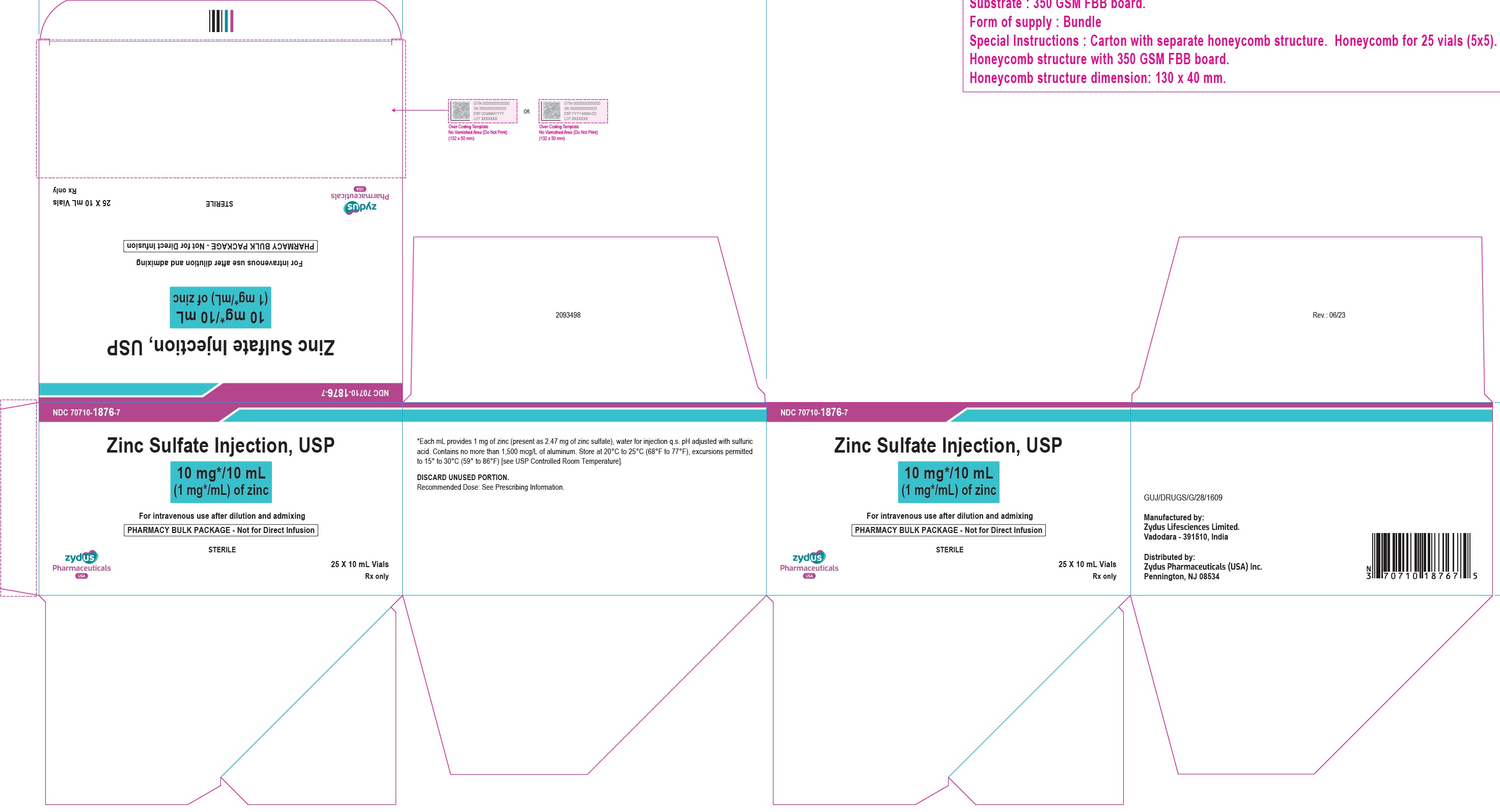
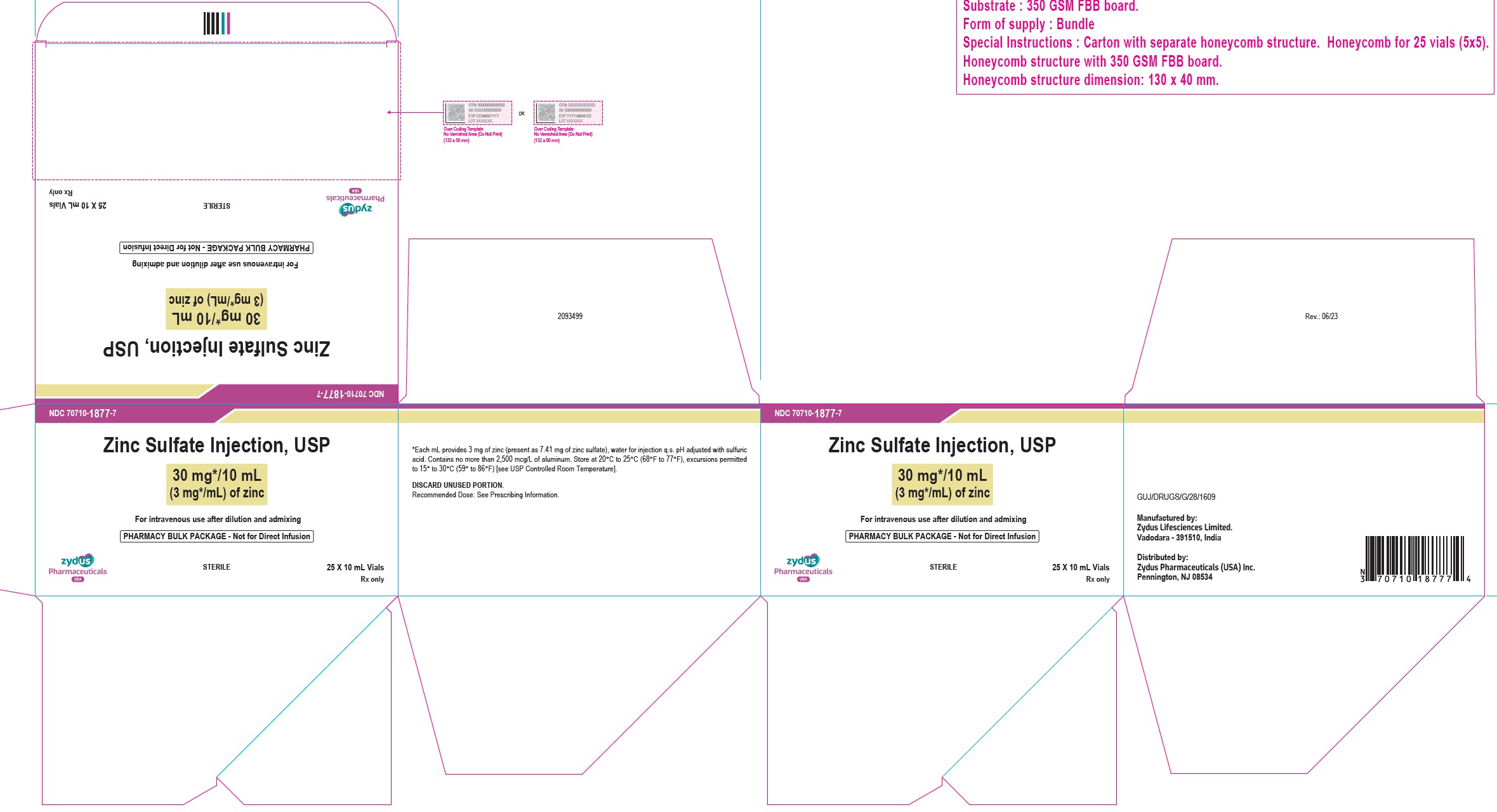
3 DOSAGE FORMS AND STRENGTHSZinc Sulfate Injection, USP: 10 mg/10 mL (1 mg/mL) of zinc as a clear, colorless solution in a 10 mL Pharmacy Bulk Package vial. 30 mg/10 mL (3 mg/mL) of zinc as a clear, colorless solution in a ...
-
4 CONTRAINDICATIONSZinc Sulfate Injection is contraindicated in patients with known hypersensitivity to zinc [see Warnings and Precautions (5.6)].
-
5 WARNINGS AND PRECAUTIONS5.1 Pulmonary Embolism due to Pulmonary Vascular Precipitates - Pulmonary vascular precipitates causing pulmonary vascular emboli and pulmonary distress have been reported in patients receiving ...
-
6 ADVERSE REACTIONSNo zinc-related adverse reactions have been reported in clinical studies or post-marketing reports in patients receiving intravenously administered parenteral nutrition solutions containing zinc ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Administration of the approved recommended dose of Zinc Sulfate Injection in parenteral nutrition is not expected to cause major birth defects, miscarriage, or ...
-
10 OVERDOSAGEThere are reported cases of overdosage with intravenous zinc in parenteral nutrition: Seven adult patients received an inadvertent overdosage of 50 mg to 75 mg elemental zinc per day in ...
-
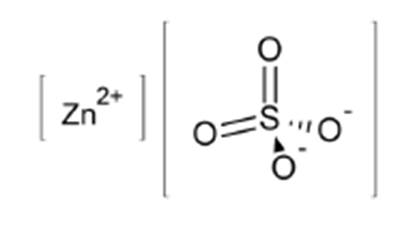
11 DESCRIPTIONZinc Sulfate Injection, USP is a sterile, non-pyrogenic, clear, colorless, and odorless solution intended for use as a trace element and an additive to intravenous solutions for parenteral ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Zinc is an essential trace element. Zinc functions as a cofactor of various enzymes including DNA polymerases, RNA polymerases, alcohol dehydrogenase, and alkaline ...
-
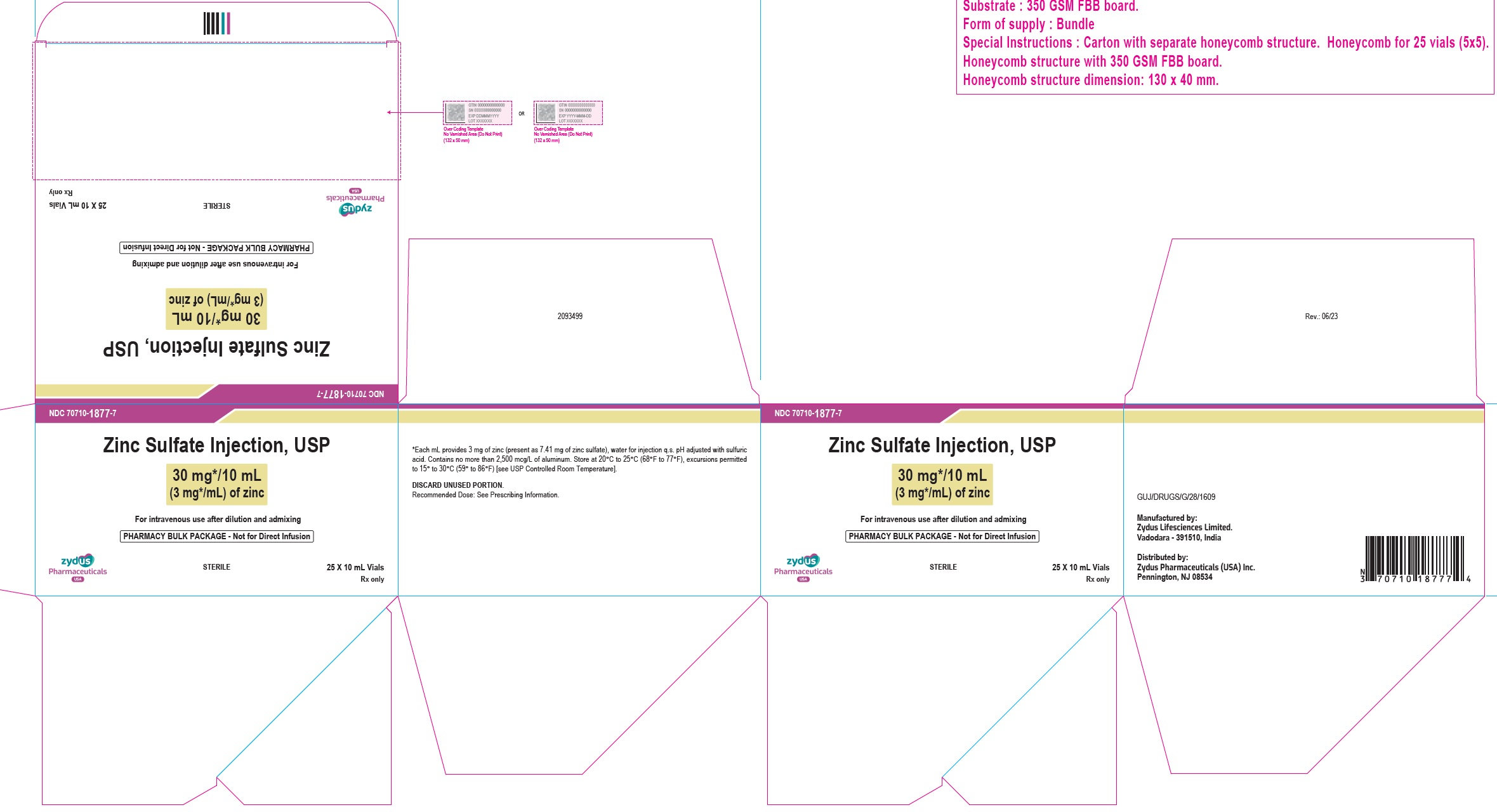
16 HOW SUPPLIED/STORAGE AND HANDLINGZinc Sulfate Injection, USP is a clear, colorless solution supplied as: 10 mg/10 mL (1 mg/mL) of zinc in a 10 mL Pharmacy Bulk Package vial. Cartons of 25 vials (NDC 70710-1876-7). 30 mg/10 mL ...
-
17 PATIENT COUNSELING INFORMATIONInform patients, caregivers or home healthcare providers of the following risks of Zinc Sulfate Injection: Pulmonary embolism due to pulmonary vascular precipitates [see Warnings and Precautions ...
-
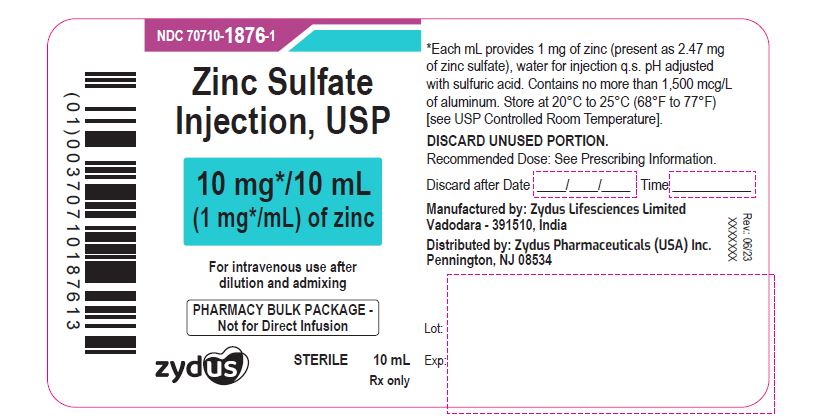
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 70710-1876-1 - Zinc Sulfate Injection, USP - 10 mg/10 mL (1 mg/mL) of zinc - For intravenous use after dilution and admixing - PHARMACY BULK PACKAGE-Not for Direct Infusion - STERILE - 10 mL - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information