Label: LEADER ICE BLUE ANALGESIC- menthol, unspecified form gel
- NDC Code(s): 70000-0260-1
- Packager: Cardinal Health, 110 dba LEADER
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
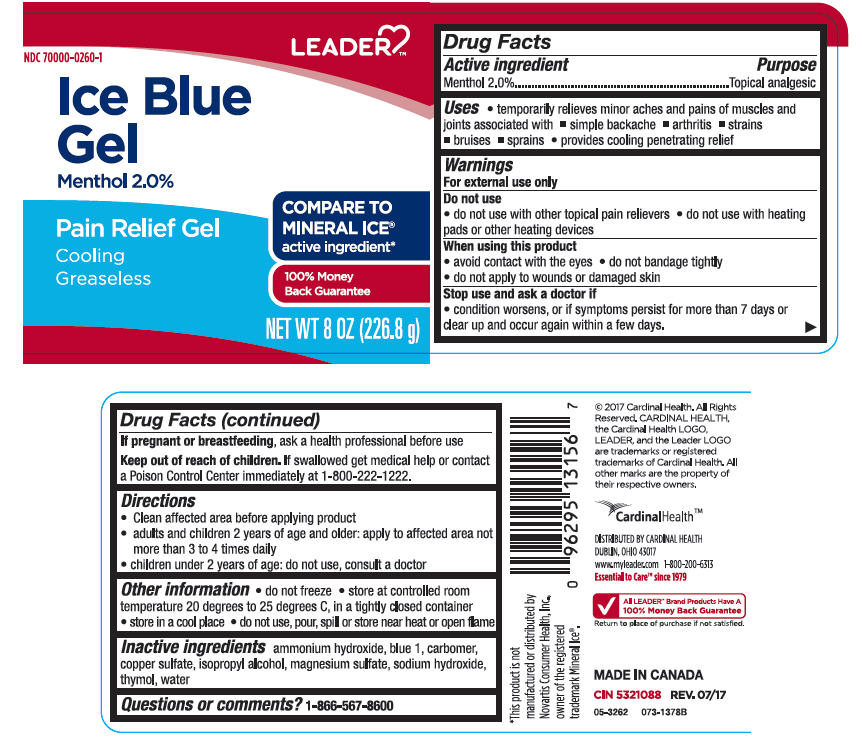
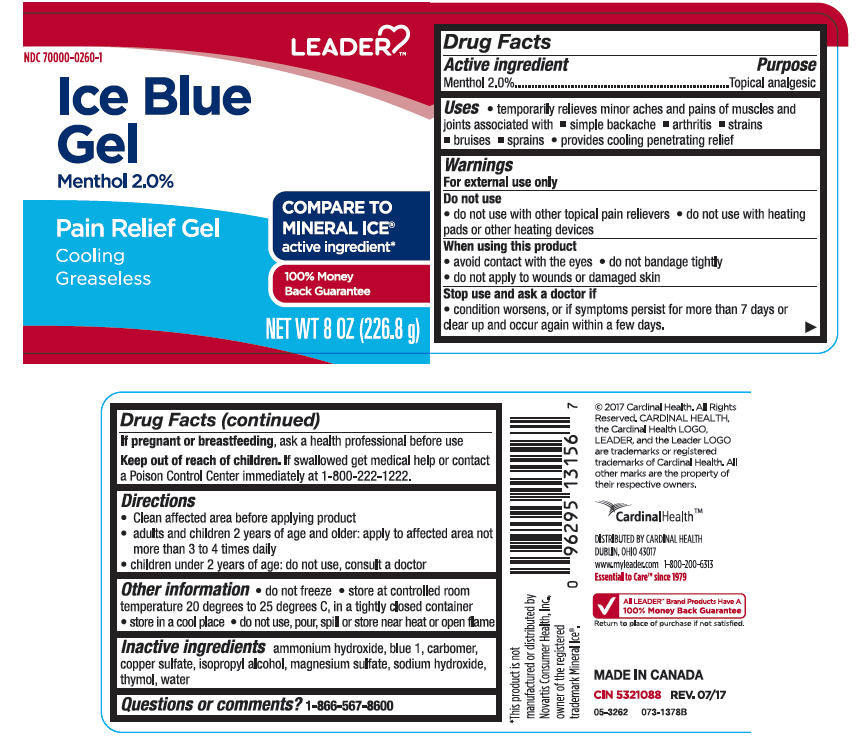
SPL UNCLASSIFIED SECTIONDrug Facts
-
Active ingredientsMenthol 2%
-
PurposeTopical Analgesic
-
Uses♦ temporarily relieves minor aches and pains of muscles and joints associated with ♦ simple backache - ♦ arthritis - ♦ strains - ♦ bruises - ♦ sprains - ♦ provides cooling penetrating ...
-
WarningsFor external use only - Do not use - ♦ do not use with other topical pain relievers - ♦ do not use with heating pads or other heating devices - When using this product - ♦ avoid contact with the ...
-
Directions♦ Clean affected area before applying product - ♦ adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily - ♦ children under 2 years of age: do not use ...
-
Other information♦ do not freeze - ♦ store at controlled room temperature 20 degrees to 25 degrees C, in a tightly closed container - ♦ store in a cool place - ♦ do not use, pour, spill or store near heat or open ...
-
Inactive ingredients Ammonium Hydroxide, Blue 1, Carbomer, Copper Sulfate, Isopropyl Alcohol, Magnesium Sulfate, Sodium Hydroxide, Thymol, Water.
-
Questions or comments?1-866-567-8600
-
SPL UNCLASSIFIED SECTIONDISTRIBUTED BY CARDINAL HEALTH - DUBLIN, OHIO 43017
-
PRINCIPAL DISPLAY PANEL - 226.8 g Jar LabelLEADER - NDC 70000-0260-1 - Ice Blue - Gel - Menthol 2.0% Pain Relief Gel - Cooling - Greaseless - COMPARE TO - MINERAL ICE® active ingredient* 100% Money - Back Guarantee - NET WT 8 OZ (226.8 g)
-
INGREDIENTS AND APPEARANCEProduct Information