General
- The safety and effectiveness of lidocaine HCl depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Standard textbooks should be consulted for ...
General
The safety and effectiveness of lidocaine HCl depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Standard textbooks should be consulted for specific techniques and precautions for various regional anesthetic procedures.
Resuscitative equipment, oxygen, and other resuscitative drugs should be available for immediate use (see
WARNINGS and
ADVERSE REACTIONS). The lowest dosage that results in effective anesthesia should be used to avoid high plasma levels and serious adverse effects. Syringe aspirations should also be performed before and during each supplemental injection when using indwelling catheter techniques. During the administration of epidural anesthesia, it is recommended that a test dose be administered initially and that the patient be monitored for central nervous system toxicity and cardiovascular toxicity, as well as for signs of unintended intrathecal administration, before proceeding. When clinical conditions permit, consideration should be given to employing local anesthetic solutions that contain epinephrine for the test dose because circulatory changes compatible with epinephrine may also serve as a warning sign of unintended intravascular injection. An intravascular injection is still possible even if aspirations for blood are negative. Repeated doses of lidocaine HCl may cause significant increases in blood levels with each repeated dose because of slow accumulation of the drug or its metabolites. Tolerance to elevated blood levels varies with the status of the patient. Debilitated, elderly patients, acutely ill patients, and children should be given reduced doses commensurate with their age and physical condition. Lidocaine HCl should also be used with caution in patients with severe shock or heart block.
Lumbar and caudal epidural anesthesia should be used with extreme caution in persons with the following conditions: existing neurological disease, spinal deformities, septicemia, and severe hypertension.
Local anesthetic solutions containing a vasoconstrictor should be used cautiously and in carefully circumscribed quantities in areas of the body supplied by end arteries or having otherwise compromised blood supply. Patients with peripheral vascular disease and those with hypertensive vascular disease may exhibit exaggerated vasoconstrictor response. Ischemic injury or necrosis may result. Preparations containing a vasoconstrictor should be used with caution in patients during or following the administration of potent general anesthetic agents, since cardiac arrhythmias may occur under such conditions.
Careful and constant monitoring of cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient’s state of consciousness should be accomplished after each local anesthetic injection. It should be kept in mind at such times that restlessness, anxiety, tinnitus, dizziness, blurred vision, tremors, depression or drowsiness may be early warning signs of central nervous system toxicity.
Since amide-type local anesthetics are metabolized by the liver, Xylocaine Injection should be used with caution in patients with hepatic disease. Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at greater risk of developing toxic plasma concentrations. Xylocaine Injection should also be used with caution in patients with impaired cardiovascular function since they may be less able to compensate for functional changes associated with the prolongation of A-V conduction produced by these drugs.
Many drugs used during the conduct of anesthesia are considered potential triggering agents for familial malignant hyperthermia. Since it is not known whether amide-type local anesthetics may trigger this reaction and since the need for supplemental general anesthesia cannot be predicted in advance, it is suggested that a standard protocol for the management of malignant hyperthermia should be available. Early unexplained signs of tachycardia, tachypnea, labile blood pressure and metabolic acidosis may precede temperature elevation. Successful outcome is dependent on early diagnosis, prompt discontinuance of the suspect triggering agent(s) and institution of treatment, including oxygen therapy, indicated supportive measures and dantrolene (consult dantrolene sodium intravenous package insert before using).
Proper tourniquet technique, as described in publications and standard textbooks, is essential in the performance of intravenous regional anesthesia. Solutions containing epinephrine or other vasoconstrictors should not be used for this technique.
Lidocaine HCl should be used with caution in persons with known drug sensitivities. Patients allergic to para-aminobenzoic acid derivatives (procaine, tetracaine, benzocaine, etc.) have not shown cross-sensitivity to lidocaine HCl.
Use in the Head and Neck Area
Small doses of local anesthetics injected into the head and neck area, including retrobulbar, dental and stellate ganglion blocks, may produce adverse reactions similar to systemic toxicity seen with unintentional intravascular injections of larger doses. Confusion, convulsions, respiratory depression and/or respiratory arrest, and cardiovascular stimulation or depression have been reported. These reactions may be due to intra-arterial injection of the local anesthetic with retrograde flow to the cerebral circulation. Patients receiving these blocks should have their circulation and respiration monitored and be constantly observed. Resuscitative equipment and personnel for treating adverse reactions should be immediately available. Dosage recommendations should not be exceeded (see
DOSAGE & ADMINISTRATION).
Information for Patients
When appropriate, patients should be informed in advance that they may experience temporary loss of sensation and motor activity, usually in the lower half of the body, following proper administration of epidural anesthesia.
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
Clinically Significant Drug Interactions
The administration of local anesthetic solutions containing epinephrine or norepinephrine to patients receiving monoamine oxidase inhibitors or tricyclic antidepressants may produce severe, prolonged hypertension.
Phenothiazines and butyrophenones may reduce or reverse the pressor effect of epinephrine.
Concurrent use of these agents should generally be avoided. In situations when concurrent therapy is necessary, careful patient monitoring is essential.
Concurrent administration of vasopressor drugs (for the treatment of hypotension related to obstetric blocks) and ergot-type oxytocic drugs may cause severe, persistent hypertension or cerebrovascular accidents.
Drug/Laboratory Test Interactions
The intramuscular injection of lidocaine HCl may result in an increase in creatine phosphokinase levels. Thus, the use of this enzyme determination, without isoenzyme separation, as a diagnostic test for the presence of acute myocardial infarction may be compromised by the intramuscular injection of lidocaine HCl.
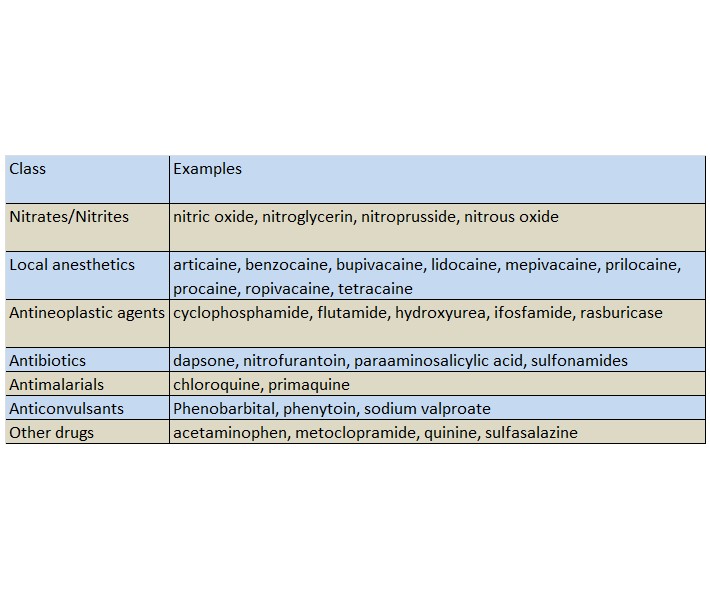
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
Examples of Drugs Associated with Methemoglobinemia:
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies of lidocaine HCl in animals to evaluate the carcinogenic and mutagenic potential or the effect on fertility have not been conducted.
Pregnancy
Teratogenic Effects: Pregnancy Category B.
Reproduction studies have been performed in rats at doses up to 6.6 times the human dose and have revealed no evidence of harm to the fetus caused by lidocaine HCl. There are, however, no adequate and well-controlled studies in pregnant women. Animal reproduction studies are not always predictive of human response. General consideration should be given to this fact before administering lidocaine HCl to women of childbearing potential, especially during early pregnancy when maximum organogenesis takes place.
Labor and Delivery
Local anesthetics rapidly cross the placenta and when used for epidural, paracervical, pudendal or caudal block anesthesia, can cause varying degrees of maternal, fetal and neonatal toxicity (see
CLINICAL PHARMACOLOGY, Pharmacokinetics and Metabolism). The potential for toxicity depends upon the procedure performed, the type and amount of drug used, and the technique of drug administration. Adverse reactions in the parturient, fetus and neonate involve alterations of the central nervous system, peripheral vascular tone and cardiac function.
Maternal hypotension has resulted from regional anesthesia. Local anesthetics produce vasodilation by blocking sympathetic nerves. Elevating the patient’s legs and positioning her on her left side will help prevent decreases in blood pressure.
The fetal heart rate also should be monitored continuously, and electronic fetal monitoring is highly advisable.
Epidural, spinal, paracervical, or pudendal anesthesia may alter the forces of parturition through changes in uterine contractility or maternal expulsive efforts. In one study, paracervical block anesthesia was associated with a decrease in the mean duration of first stage labor and facilitation of cervical dilation. However, spinal and epidural anesthesia have also been reported to prolong the second stage of labor by removing the parturient’s reflex urge to bear down or by interfering with motor function. The use of obstetrical anesthesia may increase the need for forceps assistance.
The use of some local anesthetic drug products during labor and delivery may be followed by diminished muscle strength and tone for the first day or two of life. The long-term significance of these observations is unknown. Fetal bradycardia may occur in 20 to 30 percent of patients receiving paracervical nerve block anesthesia with the amide-type local anesthetics and may be associated with fetal acidosis. Fetal heart rate should always be monitored during paracervical anesthesia. The physician should weigh the possible advantages against risks when considering a paracervical block in prematurity, toxemia of pregnancy, and fetal distress. Careful adherence to recommended dosage is of the utmost importance in obstetrical paracervical block. Failure to achieve adequate analgesia with recommended doses should arouse suspicion of intravascular or fetal intracranial injection. Cases compatible with unintended fetal intracranial injection of local anesthetic solution have been reported following intended paracervical or pudendal block or both. Babies so affected present with unexplained neonatal depression at birth, which correlates with high local anesthetic serum levels, and often manifest seizures within six hours. Prompt use of supportive measures combined with forced urinary excretion of the local anesthetic has been used successfully to manage this complication.
Case reports of maternal convulsions and cardiovascular collapse following use of some local anesthetics for paracervical block in early pregnancy (as anesthesia for elective abortion) suggest that systemic absorption under these circumstances may be rapid. The recommended maximum dose of each drug should not be exceeded. Injection should be made slowly and with frequent aspiration. Allow a 5-minute interval between sides.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when lidocaine HCl is administered to a nursing woman.
Pediatric Use
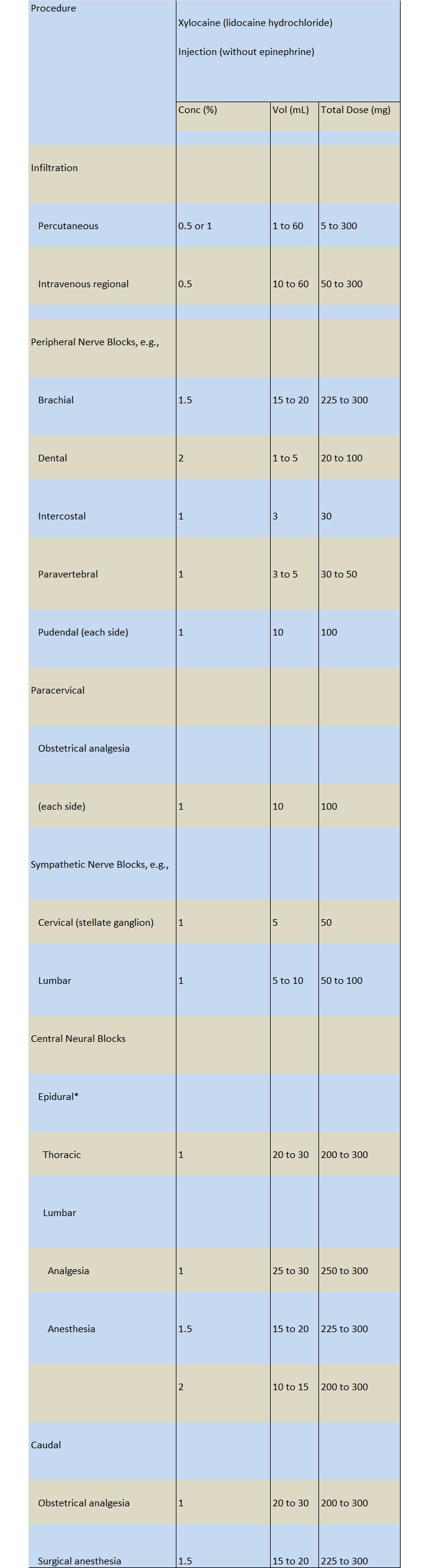
Dosages in children should be reduced, commensurate with age, body weight and physical condition (see
DOSAGE & ADMINISTRATION).
Close
 ...
...