Label: ADAPALENE cream
- NDC Code(s): 45802-453-84
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated July 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Adapalene Cream, 0.1%, contains adapalene 0.1% in an aqueous cream emulsion consisting of carbomer 934P, cyclomethicone, edetate disodium, glycerin, methyl glucose sesquistearate, methylparaben, PEG-20 methyl glucose sesquistearate, phenoxyethanol, propylparaben, purified water, squalane, and trolamine.
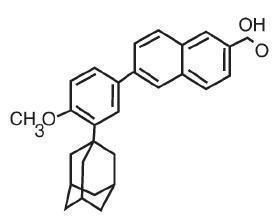
The chemical name of adapalene is 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid. It is a white to off-white powder which is soluble in tetrahydrofuran, sparingly soluble in ethanol, and practically insoluble in water. The molecular formula is C28H28O3 and molecular weight is 412.53. Adapalene is represented by the following structural formula.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Adapalene acts on retinoid receptors. Biochemical and pharmacological profile studies have demonstrated that adapalene is a modulator of cellular differentiation, keratinization, and inflammatory processes all of which represent important features in the pathology of acne vulgaris.
Mechanistically, adapalene binds to specific retinoic acid nuclear receptors but does not bind to the cytosolic receptor protein. Although the exact mode of action of adapalene is unknown, it is suggested that topical adapalene normalizes the differentiation of follicular epithelial cells resulting in decreased microcomedone formation.
Pharmacokinetics
Absorption of adapalene from Adapalene Cream, 0.1% through human skin is low. In a pharmacokinetic study with six acne patients treated once daily for 5 days with 2 grams of Adapalene Cream, 0.1% applied to 1000 cm2 of acne involved skin, there were no quantifiable amounts (limit of quantification = 0.35 ng/mL) of adapalene in the plasma samples from any patient. Excretion appears to be primarily by the biliary route.
- INDICATIONS AND USAGE
-
CLINICAL STUDIES
Two vehicle-controlled clinical studies were conducted in patients 12 to 30 years of age with mild to moderate acne vulgaris, in which Adapalene Cream, 0.1% was compared with its vehicle. Patients were instructed to apply their treatment medication once daily at bedtime for 12 weeks. In one study patients were provided with a soapless cleanser and were encouraged to refrain from using moisturizers. No other topical medications, other than Adapalene Cream, 0.1%, were to be applied to the face during the studies. Adapalene Cream, 0.1% was significantly more effective than its vehicle in the reduction of acne lesion counts. The mean percent reduction in lesion counts from baseline after treatment for 12 weeks are presented in the following table:
MEAN PERCENT REDUCTION IN LESION COUNTS FROM BASELINE TO WEEK 12 Efficacy Variable Study No. 1 Study No. 2 Adapalene
Cream, 0.1%
N=119
Cream
Vehicle
N=118Adapalene
Cream, 0.1%
N=175Cream
Vehicle
N=175Non-inflammatory lesions 34% 18% 35% 15% Inflammatory lesions 32% 17% 14% 6% Total lesions 34% 18% 30% 15% The trend in the Investigator’s global assessment of severity supported the efficacy of Adapalene Cream, 0.1% when compared to the cream vehicle.
- CONTRAINDICATIONS
-
PRECAUTIONS
General
Certain cutaneous signs and symptoms of treatment such as erythema, dryness, scaling, burning, or pruritus may be experienced with use of Adapalene Cream, 0.1%. These are most likely to occur during the first two to four weeks of treatment, are mostly mild to moderate in intensity, and usually lessen with continued use of the medication. Depending upon the severity of these side effects, patients should be instructed to reduce the frequency of application or discontinue use.
If a reaction suggesting sensitivity or chemical irritation occurs, use of the medication should be discontinued. Exposure to sunlight, including sunlamps, should be minimized during use of adapalene. Patients who normally experience high levels of sun exposure, and those with inherent sensitivity to sun, should be warned to exercise caution. Use of sunscreen products and protective clothing over treated areas is recommended when exposure cannot be avoided. Weather extremes, such as wind or cold, also may be irritating to patients under treatment with adapalene.
Avoid contact with the eyes, lips, angles of the nose, and mucous membranes. The product should not be applied to cuts, abrasions, eczematous or sunburned skin. As with other retinoids, use of “waxing” as a depilatory method should be avoided on skin treated with adapalene.
Information for Patients
Patients using Adapalene Cream, 0.1% should receive the following information and instructions:
1. This medication is to be used only as directed by the physician.
2. It is for external use only.
3. Avoid contact with the eyes, lips, angles of the nose, and mucous membranes.
4. Cleanse area with a mild or soapless cleanser before applying this medication.
5. Moisturizers may be used if necessary; however, products containing alpha hydroxy or glycolic acids should be avoided.
6. Exposure of the eye to this medication may result in reactions such as swelling, conjunctivitis, and eye irritation.
7. This medication should not be applied to cuts, abrasions, eczematous or sunburned skin.
8. Wax epilation should not be performed on treated skin due to the potential for skin erosions.
9. During the early weeks of therapy, an apparent exacerbation of acne may occur. This is due to the action of this medication on previously unseen lesions and should not be considered a reason to discontinue therapy. Overall clinical benefit may be noticed after two weeks of therapy, but at least eight weeks are required to obtain consistent beneficial effects.
Drug Interactions
As Adapalene Cream, 0.1% has the potential to produce local irritation in some patients, concomitant use of other potentially irritating topical products (medicated or abrasive soaps and cleansers, soaps and cosmetics that have a strong drying effect, and products with high concentrations of alcohol, astringents, spices or lime rind) should be approached with caution. Particular caution should be exercised in using preparations containing sulfur, resorcinol, or salicylic acid in combination with Adapalene Cream, 0.1%. If these preparations have been used, it is advisable not to start therapy with Adapalene Cream, 0.1% until the effects of such preparations in the skin have subsided.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with adapalene have been conducted in mice at topical doses of 0.4, 1.3, and 4.0 mg/kg/day, and in rats at oral doses of 0.15, 0.5, and 1.5 mg/kg/day. These doses are up to 8 times (mice) and 6 times (rats) in terms of mg/m2/day the maximum potential exposure at the recommended topical human dose (MRHD), assumed to be 2.5 grams Adapalene Cream, 0.1%, which is approximately 1.5 mg/m2 adapalene. In the oral study, increased incidence of benign and malignant pheochromocytomas in the adrenal medullas of male rats was observed.
No photocarcinogenicity studies were conducted. Animal studies have shown an increased risk of skin neoplasms with the use of pharmacologically similar drugs (e.g., retinoids) when exposed to UV irradiation in the laboratory or to sunlight. Although the significance of these studies to human use is not clear, patients should be advised to avoid or minimize exposure to either sunlight or artificial UV irradiation sources.
Adapalene did not exhibit mutagenic or genotoxic effects in vivo (mouse micronucleus test) and in vitro (Ames test, Chinese hamster ovary cell assay, mouse lymphoma TK assay) studies.
Reproductive function and fertility studies were conducted in rats administered oral doses of adapalene in amounts up to 20 mg/kg/day (up to 80 times the MRHD based on mg/m2 comparisons). No effects of adapalene were found on the reproductive performance or fertility of the F0 males or females. There were also no detectable effects on the growth, development and subsequent reproductive function of the F1 generation.
Pregnancy
Teratogenic effects
Pregnancy category C
No teratogenic effects were seen in rats at oral doses of 0.15 to 5.0 mg/kg/day adapalene (up to 20 times the MRHD based on mg/m2 comparisons). However, adapalene administered orally at doses of ≥ 25 mg/kg, (100 times the MRHD for rats or 200 times MRHD for rabbits) has been shown to be teratogenic. Cutaneous teratology studies in rats and rabbits as doses of 0.6, 2.0, and 6.0 mg/kg/day (24 times MRHD for rats or 48 times the MRHD for rabbits) exhibited on fetotoxicity and only minimal increases in supernumerary ribs in rats. There are no adequate and well-controlled studies in pregnant women. Adapalene should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Adapalene Cream, 0.1% is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 12 have not been established.
Geriatric Use
Clinical studies of Adapalene Cream, 0.1% were conducted in patients 12 to 30 years of age with acne vulgaris and therefore did not include subjects 65 years and older to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
ADVERSE REACTIONS
In controlled clinical trials, local cutaneous irritation was monitored in 285 acne patients who used Adapalene Cream, 0.1% once daily for 12 weeks. The frequency and severity of erythema, scaling, dryness, pruritus and burning were assessed during these studies. The incidence of local cutaneous irritation with Adapalene Cream, 0.1% from the controlled clinical studies is provided in the following table:
Incidence of Local Cutaneous Irritation with Adapalene Cream, 0.1% from Controlled Clinical Studies (N=285) None Mild Moderate Severe Erythema 52% (148) 38% (108) 10% (28) ‹1% (1) Scaling 58% (166) 35% (100) 6% (18) ‹1% (1) Dryness 48% (136) 42% (121) 9% (26) ‹1% (2) Pruritus (persistent) 74% (211) 21% (61) 4% (12) ‹1% (1) Burning/Stinging (persistent) 71% (202) 24% (69) 4% (12) ‹1% (2) Other reported local cutaneous adverse events in patients who used Adapalene Cream, 0.1% once daily included: sunburn (2%), skin discomfort-burning and stinging (1%) and skin irritation (1%). Events occurring in less than 1% of patients treated with Adapalene Cream, 0.1% included: acne flare, dermatitis and contact dermatitis, eyelid edema, conjunctivitis, erythema, pruritus, skin discoloration, rash, and eczema.
-
OVERDOSAGE
Adapalene Cream, 0.1% is intended for cutaneous use only. If the medication is applied excessively, no more rapid or better results will be obtained and marked redness, scaling, or skin discomfort may occur. The acute oral toxicity of Adapalene Cream, 0.1% in mice and rats is greater than 10 mL/kg. Chronic ingestion of the drug may lead to the same side effects as those associated with excessive oral intake of Vitamin A.
-
DOSAGE AND ADMINISTRATION
Adapalene Cream, 0.1% should be applied to affected areas of the skin, once daily at nighttime. A thin film of the cream should be applied to the skin areas where acne lesions appear, using enough to cover the entire affected areas lightly. A mild transitory sensation of warmth or slight stinging may occur shortly after the application of Adapalene Cream, 0.1%.
- HOW SUPPLIED
- STORAGE
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - CARTON
For topical use only. Not for ophthalmic, oral, or intravaginal use.
Usual Dosage: Apply a thin film once a day at nighttime to affected areas. See package insert for complete prescribing information.
Contains: adapalene 0.1% in an aqueous cream emulsion consisting of carbomer 934P, cyclomethicone, edetate disodium, glycerin, methyl glucose sesquistearate, methylparaben, PEG-20 methyl glucose sesquistearate, phenoxyethanol, propylparaben, purified water, squalane, and trolamine.
Store at controlled room temperature 68-77°F (20-25°C) excursions permitted between 59 and 86°F (15-30°C). Protect from freezing.
Rx Only
NDC 45802-453-84
Adapalene Cream, 0.1%
NET WT 45 g
PadagisMade in Canada
Manufactured by G Production Inc
Baie d'Urfé, QC, H9X 3S4 Canada
Distributed By
Padagis
Allegan, MI 49010 • www.padagis.com
P57355-0 9A184 RC C5 Rev. 12/2022
-
INGREDIENTS AND APPEARANCE
ADAPALENE
adapalene creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:45802-453 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADAPALENE (UNII: 1L4806J2QF) (ADAPALENE - UNII:1L4806J2QF) ADAPALENE 0.1 g in 100 g Inactive Ingredients Ingredient Name Strength Carbomer Homopolymer Type B (allyl Pentaerythritol Crosslinked) (UNII: HHT01ZNK31) CYCLOMETHICONE (UNII: NMQ347994Z) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SQUALANE (UNII: GW89575KF9) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45802-453-84 1 in 1 CARTON 10/27/2010 1 45 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA020748 10/26/2010 Labeler - Padagis Israel Pharmaceuticals Ltd (600093611) Registrant - Padagis Israel Pharmaceuticals Ltd (600093611) Establishment Name Address ID/FEI Business Operations G Production Inc. 251676961 manufacture(45802-453)