Label: PYRIDOXINE HCI injection, solution
- NDC Code(s): 51662-1410-1, 51662-1410-2, 51662-1410-3
- Packager: HF Acquisition Co LLC, DBA HealthFirst
- This is a repackaged label.
- Source NDC Code(s): 63323-180
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED
-
DESCRIPTION
Pyridoxine Hydrochloride Injection, USP is a sterile solution of pyridoxine hydrochloride in Water for Injection. Each mL contains 100 mg pyridoxine hydrochloride and 0.5% chlorobutanol anhydrous (chloral deriv.). pH adjusted with sodium hydroxide if necessary (2.0 to 3.8).
Pyridoxine hydrochloride is a colorless or white crystal or a white crystalline powder. One gram dissolves in 5 mL of water. It is stable in air and is slowly affected by sunlight.
The chemical name is 2-methyl-3-hydroxy-4,5-bis (hydroxymethyl) pyridine hydrochloride.
The structural formula is:
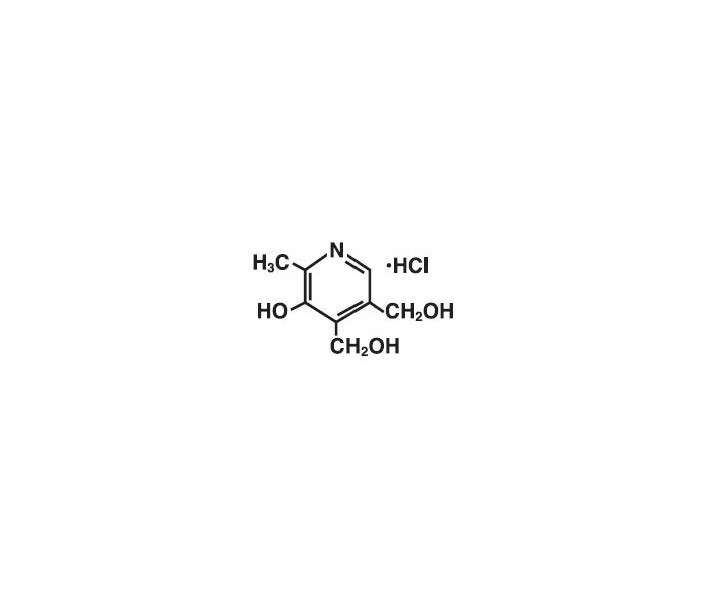
-
CLINICAL PHARMACOLOGY
Natural substances that have vitamin B6 activity are pyridoxine in plants and pyridoxal or pyridoxamine in animals. All 3 are converted to pyridoxal phosphate by the enzyme pyridoxal kinase. The physiologically active forms of vitamin B6 are pyridoxal phosphate (codecarboxylase) and pyridoxamine phosphate. Riboflavin is required for the conversion of pyridoxine phosphate to pyridoxal phosphate.
Vitamin B6 acts as a coenzyme in the metabolism of protein, carbohydrate, and fat. In protein metabolism, it participates in the decarboxylation of amino acids, conversion of tryptophan to niacin or to serotonin (5-hydroxtryptamine), deamination, and transamination and transulfuration of amino acids. In carbohydrate metabolism, it is responsible for the breakdown of glycogen to glucose-1-phosphate.
The total adult body pool consists of 16 to 25 mg of pyridoxine. Its half-life appears to be 15 to 20 days. Vitamin B6 is degraded to 4-pyridoxic acid in the liver. This metabolite is excreted in the urine.
The need for pyridoxine increases with the amount of protein in the diet. The tryptophan load test appears to uncover early vitamin B6 deficiency by detecting xanthinurea. The average adult minimum daily requirement is about 1.25 mg. The ‘‘Recommended Dietary Allowance’’ of the National Academy of Sciences is estimated to be as much as 2.2 mg for adults and 2.5 mg for pregnant and lactating women. The requirements are more in persons having certain genetic defects or those being treated with isonicotinic acid hydrazide (INHJ) or oral contraceptives.
-
INDICATIONS & USAGE
Pyridoxine Hydrochloride Injection is effective for the treatment of pyridoxine deficiency as seen in the following:
Inadequate dietary intake.
Drug-induced deficiency, as from isoniazid (INH) or oral contraceptives.
Inborn errors of metabolism, e.g., vitamin B6 dependent convulsions or vitamin B6 responsive anemia.
The parenteral route is indicated when oral administration is not feasible as in anorexia, nausea and vomiting, and preoperative and postoperative conditions. It is also indicated when gastrointestinal absorption is impaired.
- CONTRAINDICATIONS
-
WARNINGS
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
-
PRECAUTIONS
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
- ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
-
DOSAGE & ADMINISTRATION
Pyridoxine Hydrochloride Injection may be administered intramuscularly or intravenously. In cases of dietary deficiency, the dosage is 10 to 20 mg daily for 3 weeks. Follow-up treatment is recommended daily for several weeks with an oral therapeutic multivitamin preparation containing 2 to 5 mg pyridoxine. Poor dietary habits should be corrected, and an adequate, well balanced diet should be prescribed.
The vitamin B6 dependency syndrome may require a therapeutic dosage of as much as 600 mg a day and a daily intake of 30 mg for life.
In deficiencies due to INH, the dosage is 100 mg daily for 3 weeks followed by a 30 mg maintenance dose daily.
In poisoning caused by ingestion of more than 10 g of INH, an equal amount of pyridoxine should be given — 4 g intravenously followed by 1 g intramuscularly every 30 minutes.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
-
HOW SUPPLIED
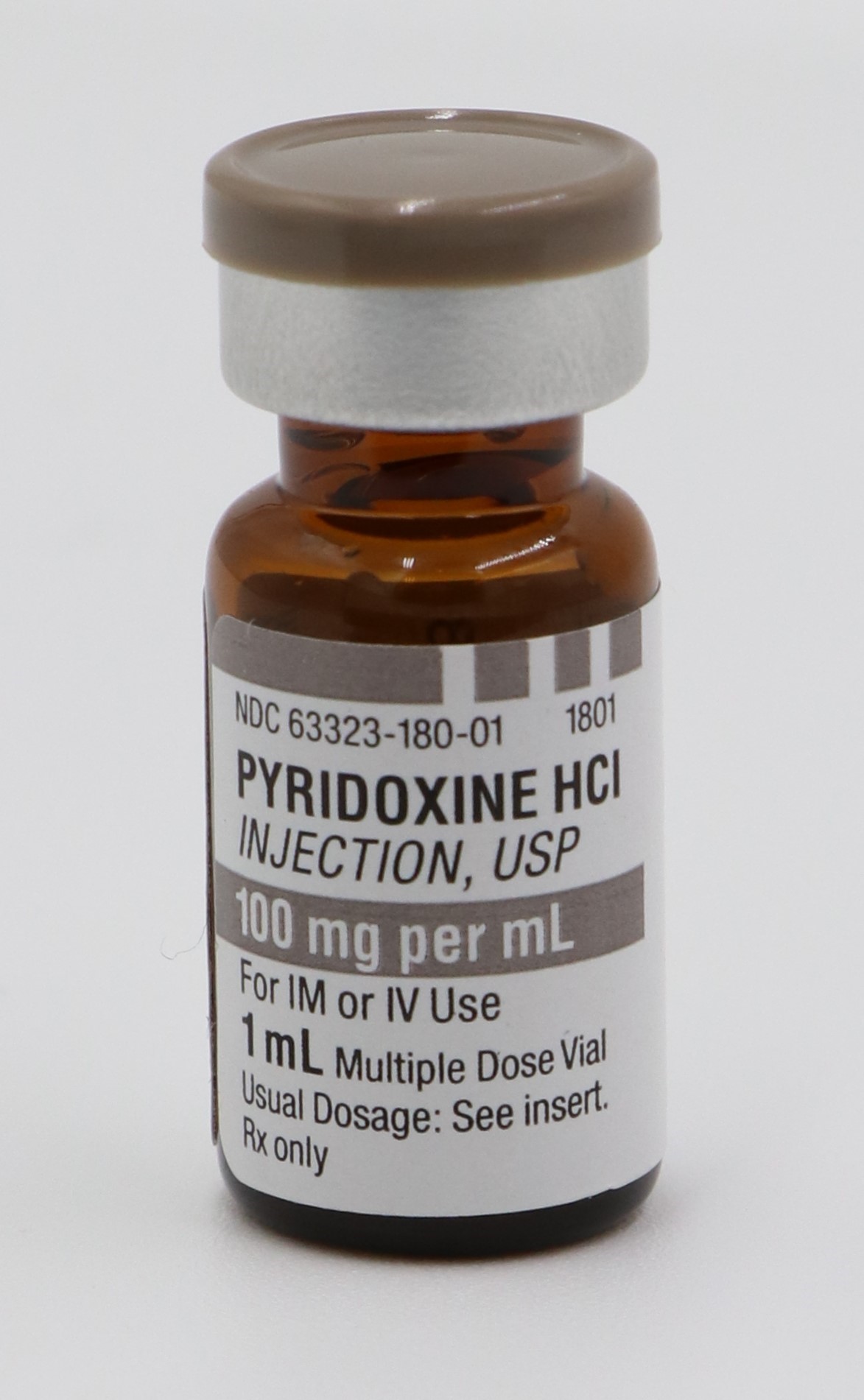
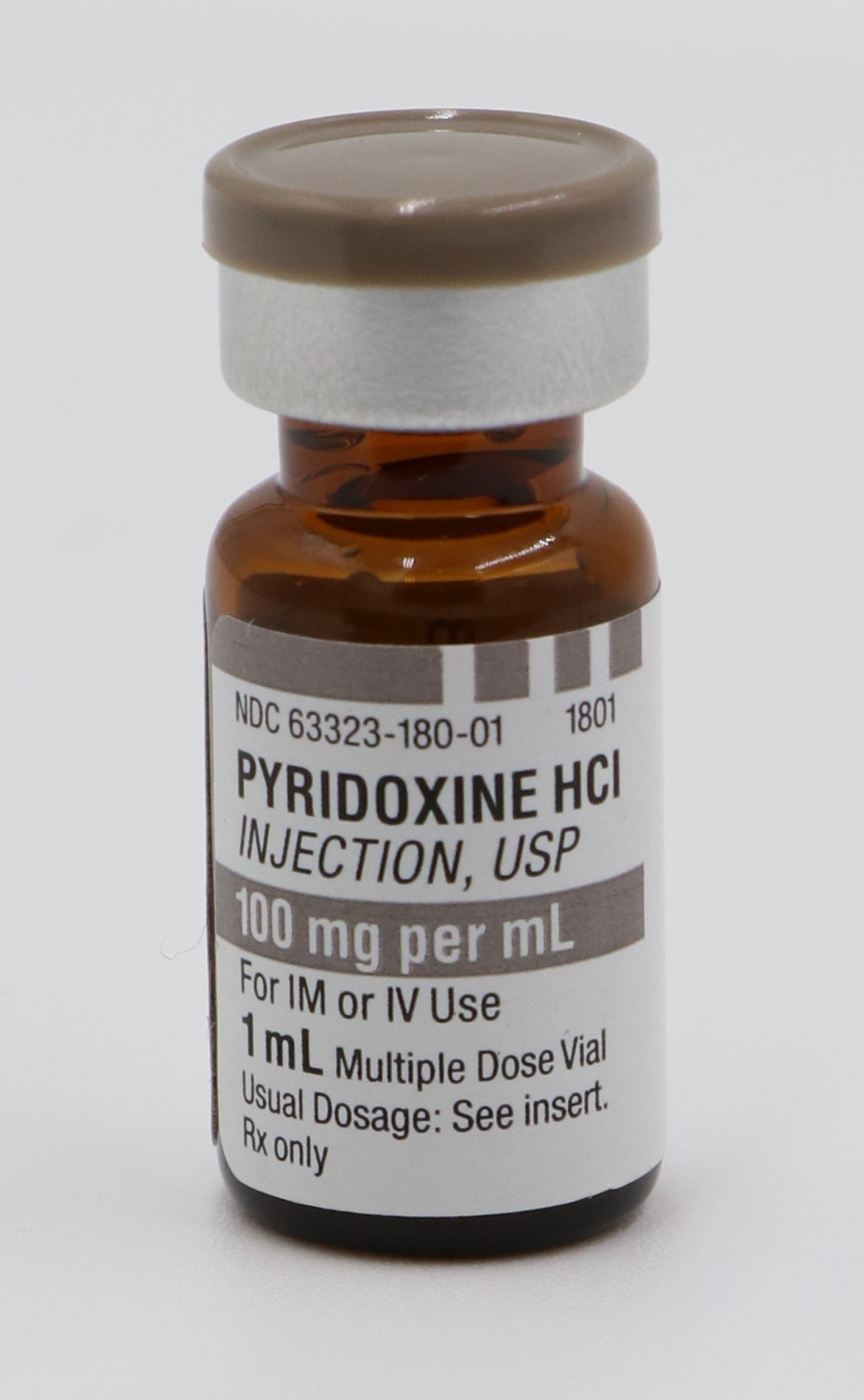
PYRIDOXINE HCI INJECTION, USP is supplied in the following dosage forms.
NDC 51662-1410-1
PYRIDOXINE HCI INJECTION, USP 100mg per mL 1mL VIALNDC 51662-1410-2
PYRIDOXINE HCI INJECTION, USP 100mg per mL 1mL VIAL, 1 VIAL PER POUCHNDC 51662-1410-3
PYRIDOXINE HCI INJECTION, USP 100mg per mL 1mL VIAL, 1 VIAL PER POUCH, 25 POUCHES PER CASEHF Acquisition Co LLC, DBA HealthFirst
Mukilteo, WA 98275PROTECT FROM LIGHT.
Use only if solution is clear and seal intact.
Sterile.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
- SPL UNCLASSIFIED
- PRINCIPAL DISPLAY PANEL, VIAL LABEL
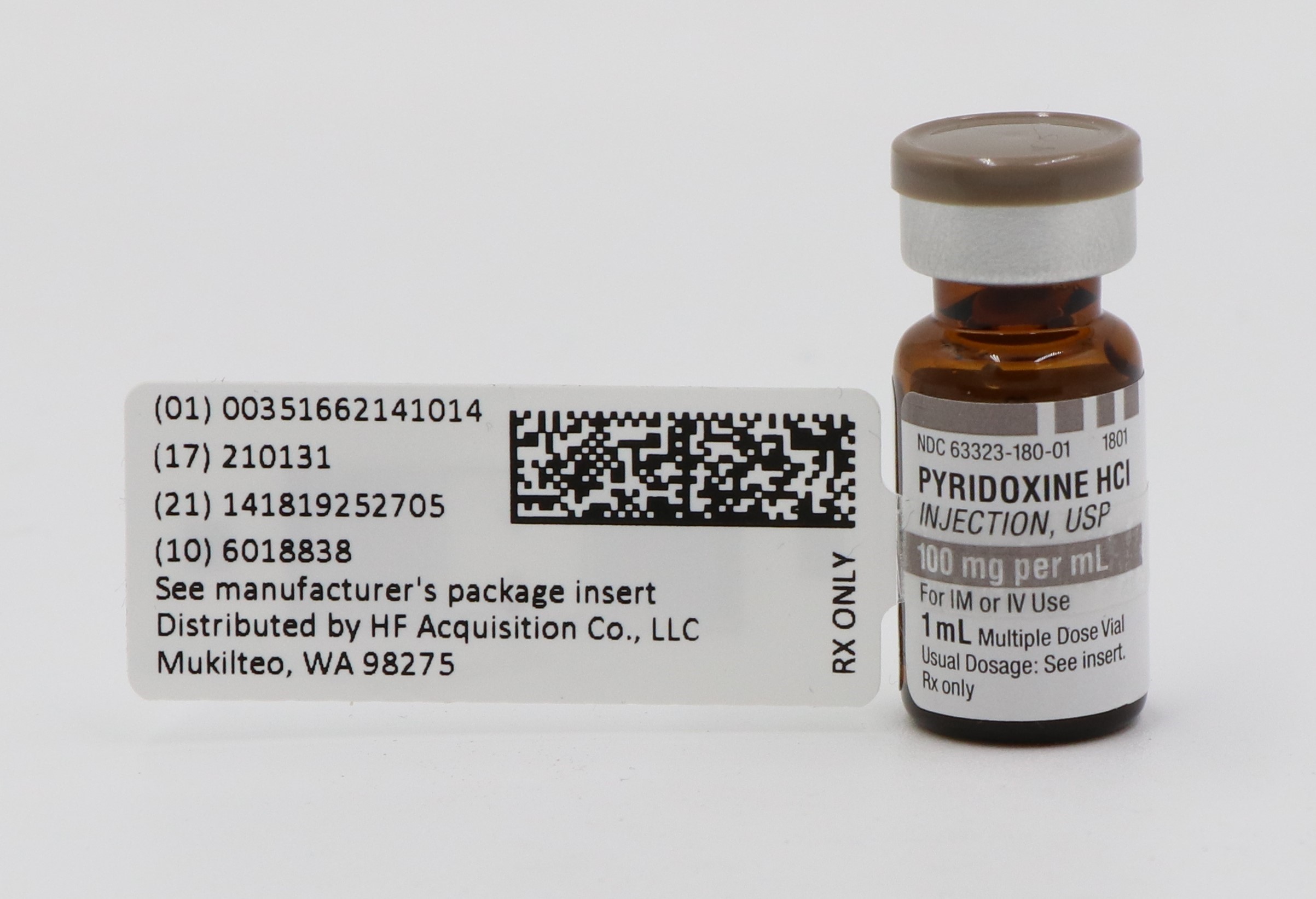
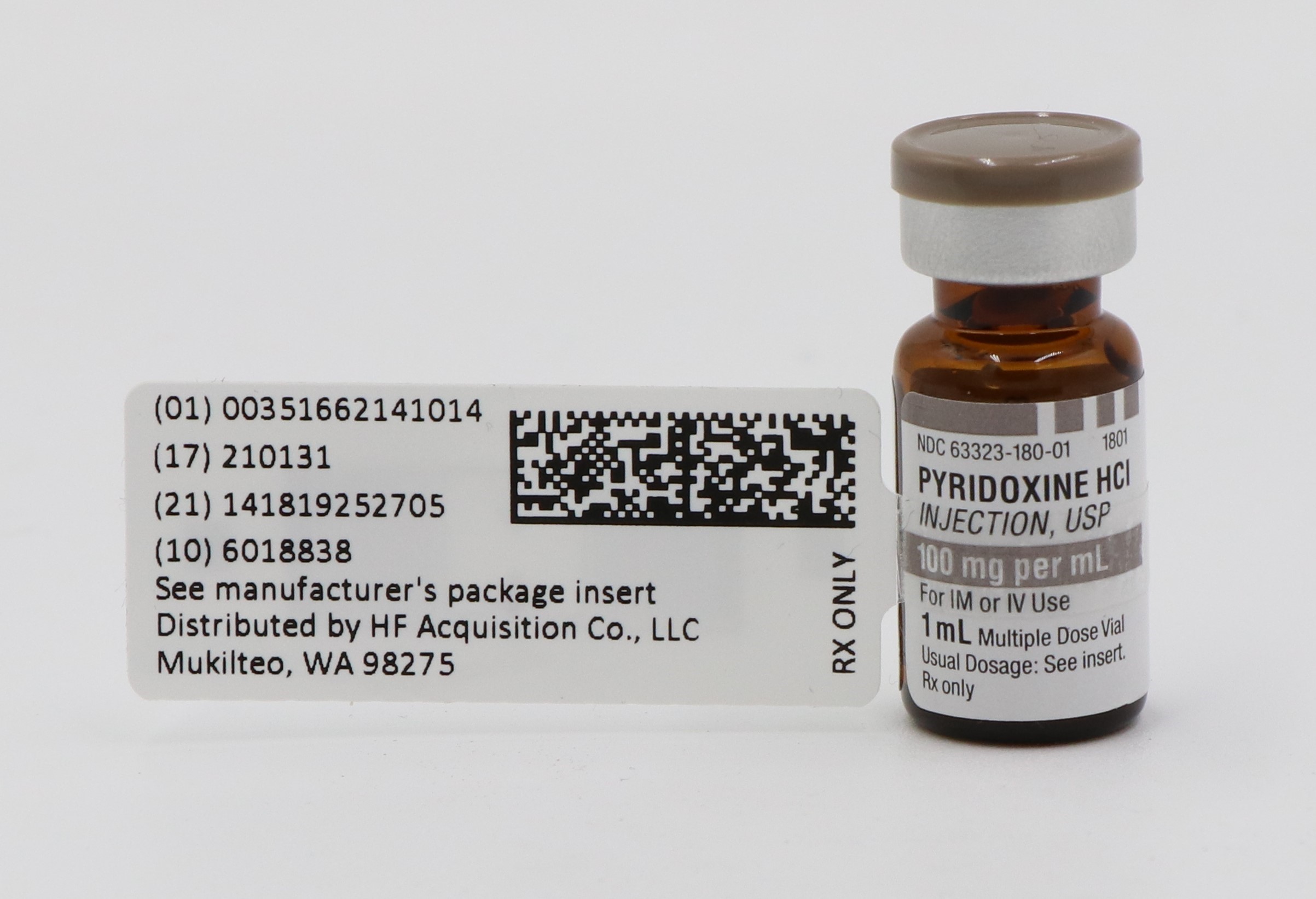
- PRINCIPAL DISPLAY PANEL, SERIALIZED LABELING
- PRINCIPAL DISPLAY PANEL - NDC 51662-1410-2 POUCH LABELING
- PRINCIPAL DISPLAY PANEL - NDC 51662-1410-3 CASE LABELING
-
INGREDIENTS AND APPEARANCE
PYRIDOXINE HCI
pyridoxine hci injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51662-1410(NDC:63323-180) Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength CHLOROBUTANOL (UNII: HM4YQM8WRC) 5 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51662-1410-1 1 mL in 1 VIAL; Type 0: Not a Combination Product 10/08/2019 2 NDC:51662-1410-3 25 in 1 CASE 12/11/2022 2 NDC:51662-1410-2 1 in 1 POUCH 2 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA080618 10/08/2019 Labeler - HF Acquisition Co LLC, DBA HealthFirst (045657305) Registrant - HF Acquisition Co LLC, DBA HealthFirst (045657305) Establishment Name Address ID/FEI Business Operations HF Acquisition Co LLC, DBA HealthFirst 045657305 relabel(51662-1410)