Label: TOPICALE XTRA- benzocaine gel
- NDC Code(s): 10733-174-01
- Packager: Medical Products Laboratories,Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Methemoglobinemia warning:
Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops pale, grey or blue colored skin (cyanosis), headache, rapid heart rate, shortness of breath, dizziness or light headedness, fatigue or lack of energy.
Contraindications:
Do not use in large quantities or over large areas of body
Do not use for Teething
Do not use in children under 2 years of ageAllergy Alert:
Do not use if you have a history of allergy to local anesthetics such as benzocaine, butacaine, procaine or other "caine" anestheticsWhen using this product
- Avoid contact with eyes
-
DOSAGE & ADMINISTRATION
Do not use more than directed.
Adults and children 12 years or older - Apply to the affected area. Allow to remain in place at least one minute and the spit out. Use up to 4 times daily or as directed by a dentist or doctor.Children 2-12 years of age - Should be supervised in the use of the product.
Children under 2 years of age - Do not use. - OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
-
INFORMATION FOR OWNERS/CAREGIVERS
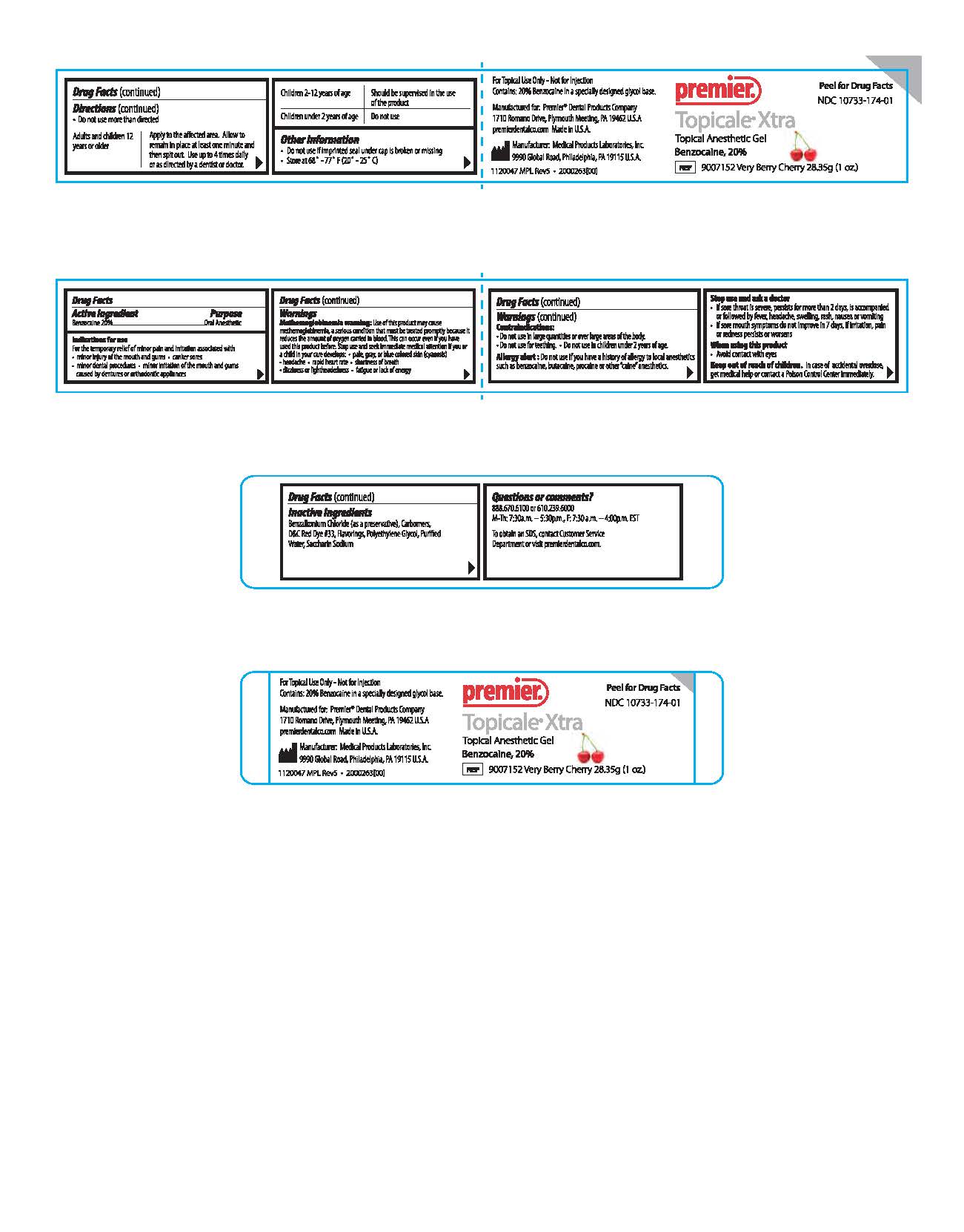
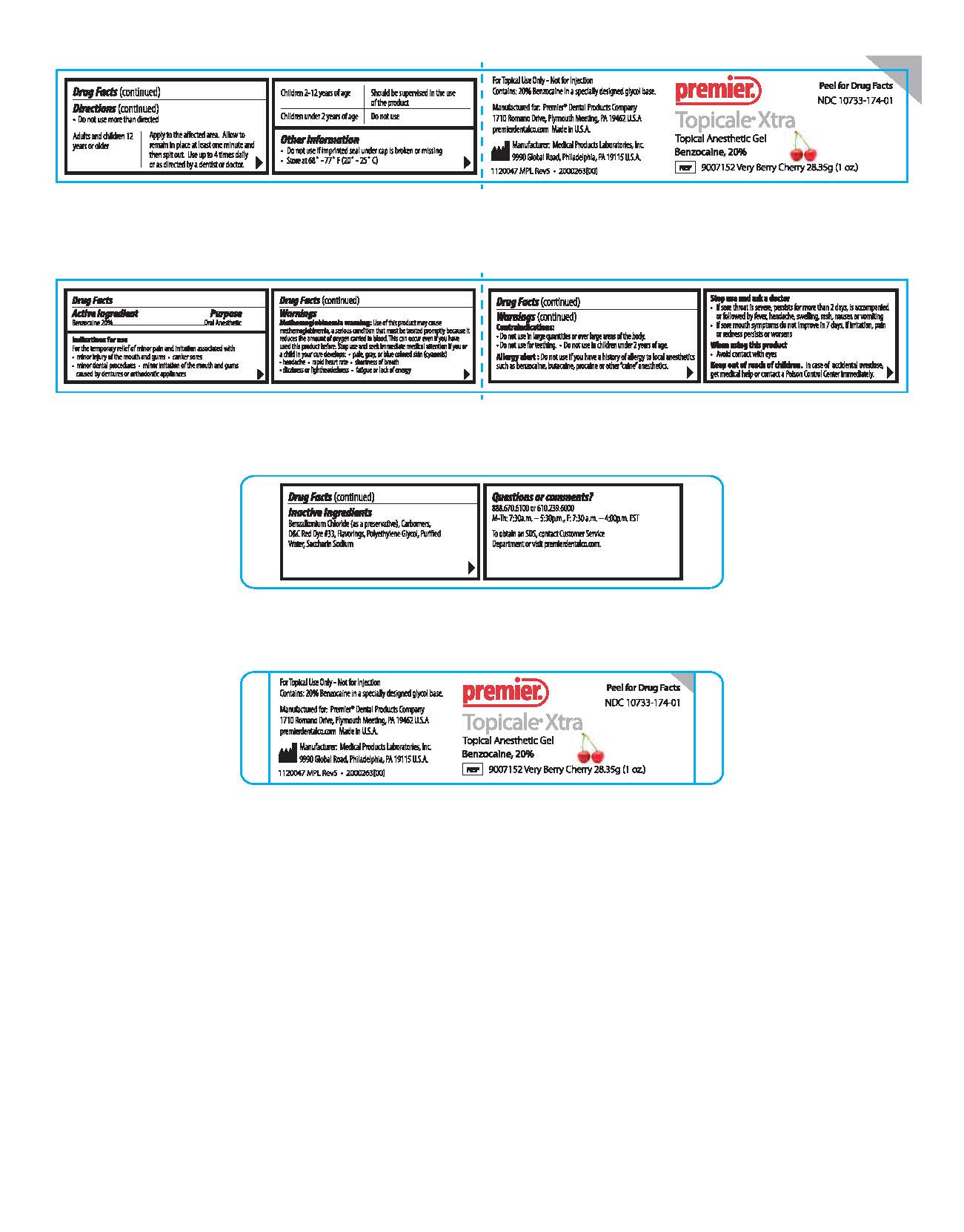
Peel for Drug Facts
NDC 10733-174-01
Premier
Topicale Xtra
Topical Anesthetic Gel
Benzocaine, 20 %
REF 9007152 Very Berry Cherry 28.35g (1 o.z.)For Topical Use only - Not for Injection
Contains: 20 % Benzocaine in a specially designed glycol base
Made in U.S.A.premierdentalco.com
Manufactured for: Premier® Dental Products Company,
1710 Romano Drive, Plymouth Meeting, PA 19462 U.S.A.
Mfg: Medical Products Laboratories, Inc.
9990 Global Road Philadelphia, PA 19115 U.S.A.
1120047 MPL Rev5 . 2000263(00)
Questions or Comments?
888.670.6100 or 610.239.6000
M-Th: 7:30a.m. - 5:30p.m., F: 7:30a.m. - 4:00p.m. EST
To obtain an SDS, contact Customer Service Department or visit premierdentalco.com. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TOPICALE XTRA
benzocaine gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10733-174 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 200 mg in 1 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) WATER (UNII: 059QF0KO0R) D&C RED NO. 33 (UNII: 9DBA0SBB0L) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL OR ALLYL SUCROSE CROSSLINKED) (UNII: K6MOM3T5YL) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Product Characteristics Color Score Shape Size Flavor MINT (Listed under NDC # 10733-175-01) , CHERRY, STRAWBERRY (Listed under NDC # 10733-176-01) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10733-174-01 28.35 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/29/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 07/29/2022 Labeler - Medical Products Laboratories,Inc. (002290302)