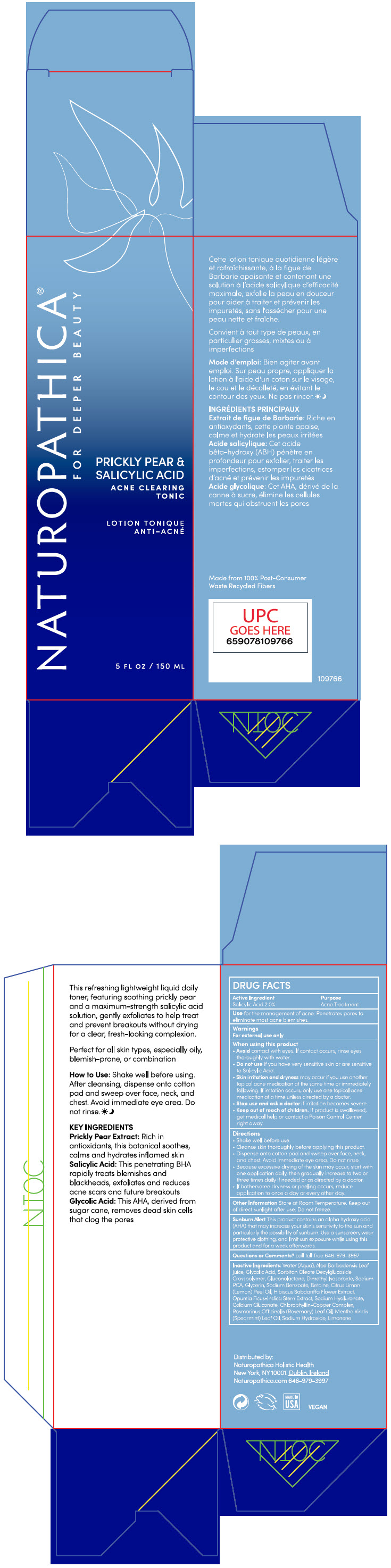
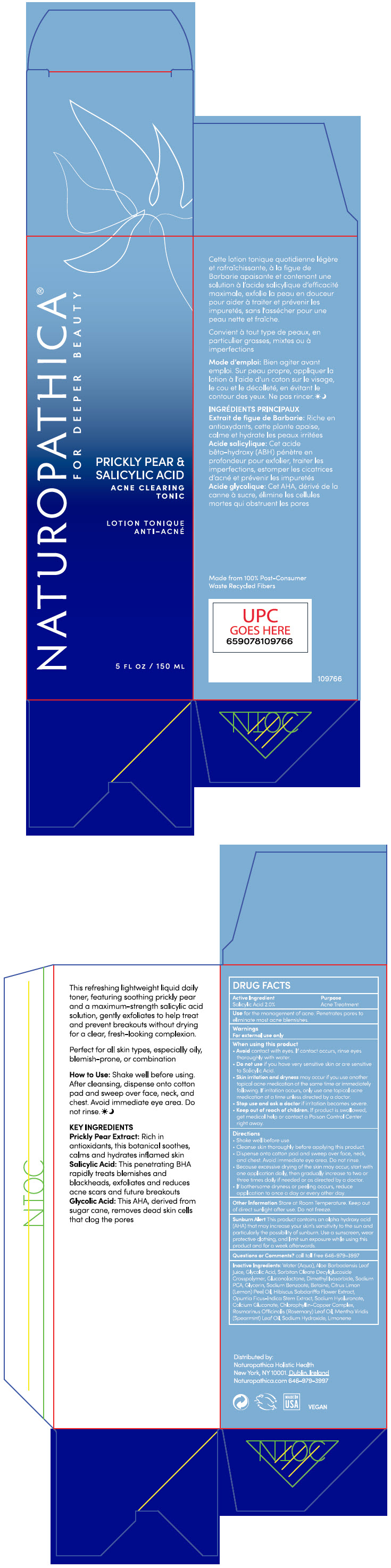
Label: PRICKLY PEAR AND SALICYLIC ACID ACNE CLEARING TONIC- salicylic acid liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 64657-003-01, 64657-003-03 - Packager: Naturopathica Holistic Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 2, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Use
-
Warnings
For external use only
When using this product
- Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
- Do not use if you have very sensitive skin or are sensitive to Salicylic Acid.
- skin irritation and dryness may occur if you use another topical acne medication at the same time or immediately following. If irritation occurs, only use one topical acne medication at a time unless directed by a doctor.
-
Directions
- Shake well before use.
- Cleanse skin thoroughly before applying this product.
- Dispense onto cotton pad and sweep over face, neck and chest. Avoid immediate eye area. Do not rinse.
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Other Information
- Sunburn Alert
-
Inactive Ingredients
Water (Aqua), Aloe Barbadensis Leaf Juice, Glycolic Acid, Sorbitan Oleate Decylglucoside Crosspolymer, Gluconolactone, Dimethyl Isosorbide, Sodium PCA, Glycerin, Sodium Benzoate, Betaine, Citrus Limon (Lemon) Peel Oil, Hibiscus Sabdariffa Flower Extract, Opuntia Ficus-Indica Stem Extract, Sodium Hyaluronate, Calcium Gluconate, Chlorophyllin-Copper Complex, Rosmarinus Officinalis (Rosemary) Leaf Oil, Mentha Viridis (Spearmint) Leaf Oil, Sodium Hydroxide, Limonene.
- Questions or Comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 150 ML Bottle Carton
-
INGREDIENTS AND APPEARANCE
PRICKLY PEAR AND SALICYLIC ACID ACNE CLEARING TONIC
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64657-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCOLIC ACID (UNII: 0WT12SX38S) GLUCONOLACTONE (UNII: WQ29KQ9POT) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) GLYCERIN (UNII: PDC6A3C0OX) SODIUM BENZOATE (UNII: OJ245FE5EU) BETAINE (UNII: 3SCV180C9W) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) HIBISCUS SABDARIFFA FLOWER (UNII: 45TGG6IU6M) OPUNTIA FICUS-INDICA STEM (UNII: MUD8892KHL) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CALCIUM GLUCONATE (UNII: SQE6VB453K) SODIUM COPPER CHLOROPHYLLIN (UNII: 1D276TYV9O) ROSEMARY OIL (UNII: 8LGU7VM393) SPEARMINT OIL (UNII: C3M81465G5) SODIUM HYDROXIDE (UNII: 55X04QC32I) LIMONENE, (+)- (UNII: GFD7C86Q1W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64657-003-01 1 in 1 CARTON 10/25/2021 1 150 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC:64657-003-03 150 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 10/25/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 10/25/2021 Labeler - Naturopathica Holistic Health (104302175)